5.2 Chemical Sedimentary Rocks
Whereas clastic sedimentary rocks are dominated by components that have been transported as solid clasts (clay, silt, sand, etc.), chemical sedimentary rocks are dominated by components that have been transported as ions in solution (Na+, Ca2+, HCO3−, etc.). There is some overlap between the two because almost all clastic sedimentary rocks contain cement formed from dissolved ions, and many chemical sedimentary rocks include some clasts. Since ions can stay in solution for tens of thousands of years (some much longer), and can travel for tens of thousands of kilometres, it is virtually impossible to relate chemical sediments back to their source rocks.
Chemical weathering and chemical sedimentary rocks
Many students confuse chemical weathering with chemical sedimentary rocks, or mistakenly assume that when and where chemical weathering is taking place, chemical sedimentary rocks will accumulate. Most ions in solution in rivers, lakes and the ocean are produced during chemical weathering, but those ions can remain in solution for millions of years, and during that time they can travel hundreds of thousands of km (yes, literally around the world, several times). They might eventually come out of solution as a result of a biological process or a change in the chemical conditions and will then become a mineral crystal that can settle to form a chemical sediment.
So, the calcium ions that are part of a calcite mud on the sea floor near Australia’s Great Barrier Reef could literally have come from anywhere on Earth (and almost certainly came from many different places), and might have been in solution for as little as a few days or for as long as tens of millions of years.
The most common chemical sedimentary rock, by far, is limestone. Others include chert, chalk, evaporites like rock gypsum and rock salt, and coal. Biological processes are important in the formation of some chemical sedimentary rocks, especially limestone, chert, and coal. For example, limestone is made up almost entirely of fragments of marine[1] organisms that manufacture calcite for their shells and other hard parts, and most chert includes at least some of the silica tests (shells) of tiny marine organisms (such as diatoms and radiolarians). Coal forms in fluvial or delta environments from decaying plant matter that accumulates in long-lasting swamps with low oxygen levels.
Chemical sedimentary rocks are classified based on their composition. As these rocks are often monomineralic, you will find that some of the same physical properties you learned about in Lab 2 can be utilized to identify chemical sedimentary rocks. For instance, rock gypsum is comprised predominantly of the mineral gypsum and can be easily identified by its hardness (H = 2, can be scratched with a fingernail).
The name of a chemical sedimentary rock can be modified by a textural term, to create a more descriptive and meaningful name. Some important textures for chemical sedimentary rocks include crystalline, oolitic, bioclastic, fossiliferous[2], and amorphous. A simplified classification chart for chemical sedimentary rocks is presented in Table 5.4.
| Composition | Texture | Distinctive Properties | Rock Name |
| Calcite (CaCO3)
*Note that all limestones will react with dilute HCl. |
Crystalline | Crystalline; fine to coarse grained | Crystalline limestone |
| Fossiliferous | Various fossil fragments well cemented together | Fossiliferous limestone | |
| Oolitic | Comprised of ooids (spheroidal particles typically <2 mm in diameter) | Oolitic limestone | |
| Bioclastic | Visible shell fragments weakly cemented together | Coquina | |
| Bioclastic | Soft rock made of microscopic shells | Chalk | |
| Quartz (SiO2) | Microcrystalline | Microcrystalline; hardness of ~7 (can scratch glass); may exhibit conchoidal fracture | Chert (note that dark coloured varieties may be called flint and red coloured varieties may be called jasper) |
| Halite (NaCl) | Crystalline | Crystalline; fine to coarse grained; commonly forms cubic crystals; tastes salty | Rock salt |
| Gypsum (CaSO4·H2O) | Crystalline | Crystalline; fine to coarse grained; hardness ~2 (can scratch with fingernail) | Rock gypsum |
| Organic material (plant fragments) | Amorphous | Black brittle rock with amorphous texture; low density | Coal |
Limestone
Almost all limestone forms in the oceans, and most of that forms on the shallow continental shelves, especially in tropical regions with coral reefs. Reefs are highly productive ecosystems populated by a wide range of organisms, many of which use calcium and bicarbonate ions in seawater to make carbonate minerals (especially calcite) for their shells and other structures. These include corals, of course, but also green and red algae, urchins, sponges, molluscs, and crustaceans. The hard parts of these organisms are eroded by waves and currents to produce carbonate fragments that accumulate in the surrounding region.
Figure 5.2.1 shows a cross-section through a typical reef in a tropical environment (normally between 40° N and 40° S). Reefs tend to form in areas with clear water (e.g., not close to the mouths of large rivers), and near the edges of steep drop-offs because the reef organisms thrive on nutrient-rich upwelling currents. As the reef builds up, it is eroded by waves and currents to produce carbonate sediments that are transported into the steep offshore fore reef area and the shallower inshore back reef area. These sediments are dominated by reef-type carbonate fragments of all sizes, including mud. In many such areas, carbonate-rich sediments also accumulate in quiet lagoons, where mud and mollusc-shell fragments predominate (Figure 5.2.2a) or in offshore areas with strong currents, where either foraminifera tests accumulate (Figure 5.2.2b) or calcite crystallizes inorganically to form ooids—spheres of calcite that form in shallow tropical ocean water with strong currents (Figure 5.2.2c). Coquina, another type of limestone, forms from shells and shell fragments mechanically broken by waves and once deposited, loosely cemented together.
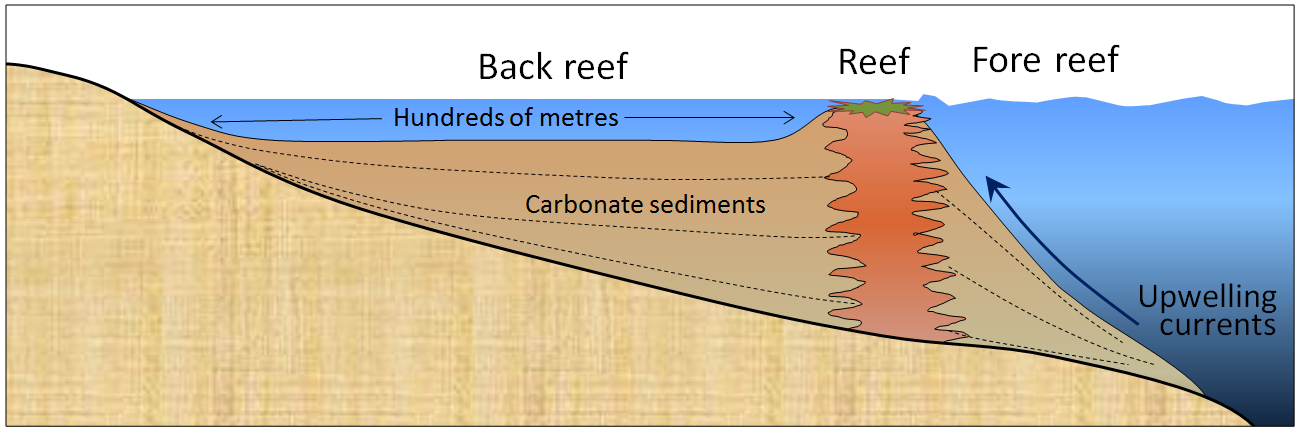

Limestone also accumulates in deeper water, from the steady rain of the carbonate shells of tiny organisms that lived near the ocean surface. For example, chalk forms from the accumulation of the calcite shells of microscopic organisms like foraminifera and coccoliths. The lower limit for limestone accumulation is around 4,000 metres. Beneath that depth, calcite is soluble so limestone does not accumulate.
Calcite can also form on land in a number of environments. Tufa forms at springs (Figure 5.2.3) and travertine forms at hot springs. Similar material precipitates within limestone caves to form stalactites, stalagmites, and a wide range of other speleothems. Tufa, travertine and speleothems make up only a tiny proportion of all limestone.

As all limestones are composed predominately of the carbonate mineral calcite, the fresh surface of a limestone will produce a strong reaction with dilute acid (i.e., will fizz vigorously). The carbonate mineral dolomite (CaMg(CO3)2), on the other hand, only produces a weak reaction with dilute acid if powdered. This is one way to distinguish the mineral dolomite from calcite, and the rock dolomite from limestone. [3] Dolomite rock is quite common (there’s a whole Italian mountain range named after it), which is surprising since marine organisms don’t make dolomite. All of the dolomite found in ancient rocks has been formed through the chemical process of magnesium replacing some of the calcium in the calcite in carbonate muds and sands. This process is known as dolomitization, and it is thought to take place where magnesium-rich water percolates through the sediments in carbonate tidal flat environments.
Chert

Not all marine organisms make their hard parts out of calcite; some, like radiolarians and diatoms, use silica (SiO2), and when they die their tiny shells (or tests) settle slowly to the bottom where they accumulate as chert. In some cases, chert is deposited along with limestone in the moderately deep ocean, but the two tend to remain separate, so chert beds within limestone are quite common (Figure 5.2.4), as are nodules, like the flint nodules within the Cretaceous chalk of southeastern England. In other situations, and especially in very deep water, chert accumulates on its own, commonly in thin beds.
Evaporites
In arid regions many lakes and inland seas have no stream outlet and the water that flows into them is removed only by evaporation. Under these conditions, the water becomes increasingly concentrated with dissolved salts, and eventually some of these salts reach saturation levels and start to crystallize (Figure 5.2.5). Although all evaporite deposits are unique because of differences in the chemistry of the water, in most cases minor amounts of carbonates start to precipitate when the solution is reduced to about 50% of its original volume. Gypsum (CaSO4·H2O) precipitates at about 20% of the original volume and halite (NaCl) precipitates at 10%. Other important evaporite minerals include sylvite (KCl) and borax (Na2B4O7·10H2O). Sylvite is mined at numerous locations across Saskatchewan from evaporites that were deposited during the Devonian (~385 Ma) when an inland sea occupied much of the region.

Coal
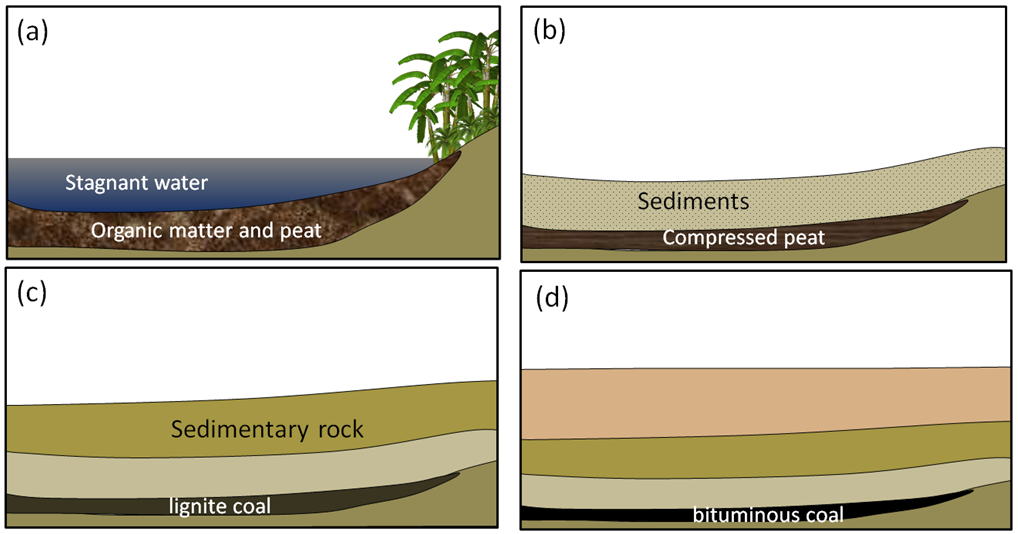
Coal, the first fossil fuel to be widely used, forms mostly on land in swampy areas adjacent to rivers and deltas in areas with humid tropical to temperate climates. The vigorous growth of vegetation leads to an abundance of organic matter that accumulates within stagnant water, and thus does not decay and oxidize. This situation, where the dead organic matter is submerged in oxygen-poor water, must be maintained for centuries to millennia in order for enough material to accumulate to form a thick layer (Figure 5.2.6a). At some point, the swamp deposit is covered with more sediment — typically because a river changes its course or sea level rises (Figure 5.2.6b). As more sediments are added, the organic matter starts to become compressed and heated. Low-grade lignite coal forms at depths between a few 100 m and 1,500 m and temperatures up to about 50°C (Figure 5.2.6c). At between 1,000 m to 5,000 m depth and temperatures up to 150°C m, bituminous coal forms (Figure 5.2.6d). At depths beyond 5,000 m and temperatures over 150°C, anthracite coal forms.
There are significant coal deposits in many parts of Canada, including the Maritimes, Ontario, Saskatchewan, Alberta, and British Columbia. In Alberta and Saskatchewan, much of the coal is used for electricity generation. Coal from the Highvale Mine, Canada’s largest, is used to feed the Sundance and Keephills power stations west of Edmonton. Almost all of the coal mined in British Columbia is exported for use in manufacturing steel.
Media Attributions
- Figures 5.2.1, 5.2.2, 5.2.3, 5.2.4, 5.2.5, 5.2.6: © Steven Earle. CC-BY-4.0.
- Figure 5.2.2c: JoultersCayOoids by Wilson44691. Public domain.
- We use the word marine when referring to salt water (i.e., oceanic) environments, and the word aquatic when referring to freshwater environments. ↵
- Note that the term "fossiliferous" can also be used to modify the name of a clastic sedimentary rock (e.g., shale, mudstone, sandstone) that contains recognizable evidence of past life. ↵
- Dolomite is both a name for a mineral and for a rock composed of the mineral dolomite (although some geologists use the term dolostone to avoid confusion). ↵

