Chapter 15: The Immune System Response to Acute and Chronic Exercise
Kevin Milne

Learning Objectives
After reading this Chapter, you should be able to:
- Describe the basic components of the immune system including the lymphatic system, the major immune organs, and the primary physiological barriers.
- List and describe the common cells and signaling molecules of the immune system.
- Compare and contrast the innate versus acquired immune systems.
- Describe the components of the immune system that are modified by exercise.
Key Terms
acute phase proteins, allergic immunity, antibodies, antigen presenting cells, antibody mediated, anti-microbial, anti-viral, apoptosis, B memory cells, C-reactive protein, cell-mediated immunity, cluster of differentiation, complement system, haematopoiesis, humoral immunity, immunology, immunosurveillance, innate/acquired or adaptive, lipopolysaccharide, lymphocytes, opsonisation, pathogens, pattern recognition receptors, phagocytes, pathogen-associated molecular patterns, plasma cells, receptors, toll-like receptors,
Case Presentation: Heavy Training can Compromise Your Immune System
Jake, who you have met earlier, and Jerry are close friends. They grew up together, were highly active through elementary and high school, and even wound up attending the same university to compete in track and field. Jerry, however, quickly found himself unable to balance his academic and athletic careers and chose to cut his university running career short. Even though he left the team, he did not stop running, and found himself enjoying the freedom to train when and where he wanted without the pressures of performance times and team practices. He also started to take up new forms of exercise, such as yoga, with a young woman he met while out on a leisurely jog. Jake, on the other hand, enjoyed a year of success and even won “Freshman of the Year” at the end of his first season. He seemed to revel in the competitive aspect of the sport and took his training to new heights over the summer, increasing his training intensity and volume such that he was without peer in practice runs. Towards the middle of the first semester in second year, there came to be a dramatic difference in the 2 young men. Jerry’s grades improved so that he was no longer in danger of being kicked out of his program, he noticed his runs were feeling easier, and his attitudes about life and health were at all-time highs. Jake, on the other hand, was still the top sophomore runner, but he never approached his performance times of the year before, and as the season progressed his performance seemed to decline every week. Jake assumed this was a function of inadequate training and reasoned that if he could only train harder and longer, he would regain some of his first-year prowess. Unfortunately, over the course of the year Jake found himself sick more often than he could remember and this significantly hindered his ability to train and perform. Even though they were roommates, Jerry seemed to enjoy a sort of immunity from the colds and flues that plagued Jake all year. One day when Jerry was heading out to the yoga studio with his girlfriend, he noticed Jake sniffling away on their beat-up dormitory lounge chair.
“Don’t worry, Jake,” he shouted just before leaving the cramped dorm room. “I’m sure you’ll be better tomorrow.”
What is it about exercise that can cause a young varsity athlete to become confined to his “beat-up old dormitory couch”, seemingly more susceptible to minor “colds and flues” as Jake described them? Further, what is it about exercise in other forms that actually appears to fortify the body against a host of potential stressors? Answers to these questions require an understanding of the immune system and the strategies employed to protect your body against disease and infection. In the next chapter, we will first learn about the important components of the human immune system including the transport vessels, cells, chemical messengers, and the functional integration of these different components to make up the fast acting innate and slow acting but powerful acquired branches of the immune system. Then, you will be introduced to how exercise affects the immune system with special emphasis on the importance of exercise dose (intensity and duration). Knowledge of these effects can be used by athletes and recreationally active individuals alike in an attempt to keep their complex but vital immune systems as healthy as possible. Small organisms and chemical molecules that originate from outside the body can initiate massive physiological defense responses when they enter our interior environment. This response is a necessity for life, but, depending on the stress, can become an energy and resource glutton and render the rest of the body almost helpless against the physical stresses that present themselves every day such as getting up and walking. Think about your energy levels when you’ve had a cold or flu like Jake in the example above. Again, depending on the severity of the sickness, exercise or movement is likely the furthest thing from your mind. This fatigue and lethargy is not typically a result of the foreign invader itself, but rather directly related to your body devoting energy to the immune system to rid yourself of the harmful invader. In a common but extreme example of the importance of the immune system, the human immunodeficiency virus (HIV) causes acquired immunodeficiency syndrome (AIDS) by destroying a vital component of the immune response, helper T Cells, and in so doing renders the body defenseless against the onslaught of bacterial and viral invaders we normally face each day. In fact, most individuals unsuccessfully treated against HIV usually succumb to AIDS opportunistic infections (Collaboration 2010) such as Pneumocystis pneumonia (PCP), a potentially fatal fungal infection that you likely carry in your lungs right now but which your healthy immune system can easily keep under control.
—-
Introduction
The Nobel Prize in Physiology and Medicine in 1908 was given jointly to doctors Ilya Mechnikov and Paul Ehrlich as pioneers in the study of the immune system. Dr. Mechnikov noticed that groups of cells that could engulf and devour foreign bodies would congregate at a puncture site in the skin of sea star larvae. Meanwhile, Dr. Ehrlich theorized that cells, when invaded by a toxin releasing substance, possess the capability to neutralize these toxins by producing specific receptors to the toxins. Dr. Mechnikov went on to name those specialized cells phagocytes (from the Greek meaning devouring cells) and Dr. Ehrlich’s side chain receptors form the basis for how we now understand antibodies to function. While they did not possess the capabilities we now do, their findings were also the bases behind the inherent and acquired immune responses that we now know are much more complex.
We live in a world of microbes whose primary biological purpose, much like our own, is to propagate the survival of their genetic information. Unfortunately for us, we and other animals are excellent incubators for microbes to thrive, develop, and achieve their life’s purpose with great success. However, it is to our benefit that many of these microbes can achieve this goal without mortal consequences to us. In fact, we co-exist with several bacteria that aid in the proper function of our physiology. For example, it is estimated that there are approximately 1012 microorganisms and their associated viruses per gram of contents that line the intestinal walls and are necessary for proper digestion and protection (Garrett et al. 2010). For example, it is estimated that there are approximately 1012 microorganisms and their associated viruses per gram of contents that line the intestinal walls and are necessary for proper digestion and protection (Garrett et al. 2010). However, it is important to be aware of the fact that there are a significant number of harmful pathogens (any agents that cause infection or disease) that require a continual upgrade of our body’s defense throughout evolution. This challenge has been referred to as a biological arms race between us and the multitude of the ever-evolving pathogens that fill our environment. The body is a well-fortified system with very limited access to what Dr. Claude Bernard, a prominent French physiologist and one of the forefathers of the concept of homeostasis, described as the milieu interieur (internal environment or fluids that surround all of our cells). It is easy to imagine, however, that when dealing with the likely thousands of attacks the body has to deal with every day, there are bound to be at least a few perpetrators that penetrate into our internal environment. As a consequence, and thankfully for us, our bodies employ multiple levels of defense that include physical barriers and mucosae (mucus membranes), non-specific chemical and cellular defenses, and specific systems targeted towards learned and recognized threats to fend off invading pathogens. Combined, these defenses make up what is known as the immune system.
—-
Highlight Feature: Edward Jenner and the Smallpox Vaccination
Before anything was known about antibodies and phagocytes, Edward Jenner, an English country doctor, cleverly introduced to the world what we now know as immunization. Jenner, who himself underwent variolation against smallpox at a very young age (i.e. deliberate infection with smallpox in an effort to protect against full blown smallpox later on), observed, as did many others, that milkmaids who suffered from cowpox (a bovine variant of the smallpox disease) were protected against smallpox. Because cowpox was symptomatically different than the more deadly smallpox, these milkmaids benefited from protection against smallpox without any of the side effects experienced in variolation. Jenner, using this knowledge, inoculated (injected) cowpox into the arm of a young boy. The boy became mildly ill with cowpox and then recovered. Several years later, he introduced smallpox to the young boy. Lucky for the boy that Jenner’s theory proved correct, he did not succumb to it. This was the first indication of vaccination (from the Latin word vacca for cow), and subsequently adopted by Louis Pasteur for the immunization against any disease. Most of us have undergone several vaccinations in infancy which required only a few doses to make us immune to what would have otherwise been life threatening diseases. This fact speaks to the remarkable memory of the immune system.
—-
The Immune System and Immunology
The word “immune” stems from the Latin immunis (im-not; munus-duty). It literally means exemption from military service, taxes or other public services, but has taken on more general (“you are immune from further persecution” or “immune from being voted off the island”) and medically specific (“immune from disease”) meanings. The immune system actually consists of the second and subsequent lines of defense in our fortified biological systems. In the true sense of the word “system”, these defenses employ a coordinated effort of different organs, cells, and molecules that function to keep us healthy. Likely one of the most important concepts in the field of immunology is that the body must be able to distinguish the billions of cells and molecules that make up each individual’s own body from an almost endless possibility of foreign invaders. As such, immunology has been defined as the science of the selective differentiation of self from non-self (Klein 1999). How does your body recognize the bacterium that causes strep throat (group A streptococcus) and destroy it, but not set the same defenses against the cells of the upper respiratory tract where these bacteria are most likely to infect? This concept is described in further detail in Highlight Feature 15-2 and highlights the problems that can occur when this distinction is not clearly made. Nonetheless, it does allude to the importance of the primary defense system (physical barriers) that prevent a need for immune action. In order to understand how exercise modifies immune system function, it is first important to learn about the important components of the defense system.
—–
Highlight Feature: Self Versus Non-self
In the evolution of multicellular organisms, the ability to separate what is foreign (non-self) from what is of host origin (self) became paramount. At one extreme, if your body cannot distinguish foreign cells from your own, it would be easy for bacteria to proliferate in the ripe conditions your body works so hard to maintain. Similarly, with an immune system that cannot distinguish what is of host origin, there would be indiscriminate attack of all cells, greatly taxing survival. In fact, several current diseases are manifest in the body’s altered recognition of self versus non-self. For example, several types of arthritis originate in the destruction of the synovium (i.e. lining of the joints) by the body’s immune system. Similarly, Type 1 diabetes begins with the attack of pancreatic beta cells by host immune cells. These are known as autoimmune (auto meaning “self”) diseases, and represent a difficult and active area of research in those and other diseases. The difficulty lies in the fact that in order to prevent the disease, portions of the hosts immune system must be disabled.
Sir Frank Macfarlane Burnet, an Australian virologist, formally introduced the discussion of “self” into the field of immunology. His theory of immunological tolerance stated that the cells and biological material that made up the host organism (i.e. self) were ignored or “tolerated” by the immune system, whereas the foreign, genetically alien cells (i.e. non-self) were destroyed. Burnet’s theories were central to the understanding of immune reactivity and portions of his work jointly won him and Sir Peter Brian Medawar, who experimentally studied the concept of immune tolerance, the 1960 Nobel Prize in Physiology or Medicine. In his Nobel lecture, Burnet asked a question that has intrigued immunologists since his time, “How can an immunized animal recognize the difference between an injected material like insulin from another species and its own corresponding substance?” For example, several drugs we currently inject are tolerated by our immune systems even though they are of foreign origin. Consequently, it may be more appropriate to think of the “self”, “non-self” or for that matter, immunogenic, as categories defined by the immune system that could include foreign or non-foreign structures rather than simply of the body or not. Indeed, Dr. Charles Janeway later theorized that the “self/non-self” categories were defined by the innate immune system using evolutionary conserved pathogen-associated molecular patterns. This has become known as Pattern Recognition Theory, and is generally accepted as a bridge between innate and adaptive immunity in mammals. However, Dr. Polly Matzinger proposed a theory in which the immune system does not care so much for “self/non-self”, but which is concerned with preventing insult to the bodily tissues. Having at least some recognition of “self” best allows the system to determine what is dangerous, but is not the deciding factor in generating an immune response. Nonetheless, while “self/non-self” is taught in every immunology class, the question asked by Dr. Burnet is still actively investigated to this day.
—-
Physical and Chemical Barriers
The skin acts as the first line of defense in the battle to protect the body from foreign invasion. A structural protein in skin cells, keratin, makes them highly resistant to many forms of bacterial attack. Further, sebaceous cells located predominantly in the face, head, and hairy regions of the body, secrete an acidic oily substance (sebum) that contains chemicals to inhibit and destroy many pathogens. Further, all cavities open to the exterior (remember, the respiratory, gastrointestinal, and urogenital tracts are all exposed to the internal environment) are lined with mucous membranes whose sticky mucus can trap foreign particles. This is supplemented by the mechanical actions of coughing, sneezing, crying (tears), urination, and the chemical actions of enzymes and other anti-microbial or anti-viral substances to keep invaders out. These physical, mechanical, and chemical barriers are listed in Table 15-1.
|
|
Function |
|---|---|
|
Physical Barriers |
|
|
The skin and interior epithelium |
|
|
|
|
|
Mucous membranes |
|
|
Mucus |
|
|
Gastrointestinal tract |
|
|
Urogenital tract |
|
|
Respiratory tract and lungs |
|
|
Chemical Defenses |
|
|
pH |
|
|
Enzymes |
|
|
|
|
Typically, these barriers are sufficient to prevent infection, and it should be easy to see why the risk of infection rises substantially when those barriers are broken. For that reason, physical exercise in which close physical contact and the increased potential for scrapes, cuts, bruises, and bleeding to occur increases the potential for infection and transmission of respiratory and blood-borne pathogens. This presents a difficult issue for athletes, parents, coaches, and sports officials given that many individuals might not know if they are infected with a disease at the time of athletic competition. To highlight this difficulty, in 1991, one of the greatest basketball players in the world, Earvin “Magic” Johnson held a press conference to announce that he had tested positive for HIV and would retire from the National Basketball Association (NBA). However, Magic was voted into the all-star game for that season (1991-1992). Several players indicated their discontent, especially given their unawareness of how diseases were spread. Nonetheless, several physicians cleared him to play and amid large public debate, he did compete and won the MVP for that contest. While the other players’ concerns were warranted, today, several organizations present guidelines about minimizing the spread of infectious diseases during sport and one of the most notable findings is that the risk of serious blood-borne disease spreading during athletic competition is slim. A summary of the recommendations by the American Academy of Pediatrics and National Athletic Therapists Association are presented in Highlight Feature “Position Statement on HIV and Other Blood-Borne Viral Pathogens in the Athletic Setting”
—-
High-light Feature: Position Statement on HIV and Other Blood-Borne Viral Pathogens in the Athletic Setting
The American Academy of Pediatrics (www.AAP.org) has established recommendations for individuals participating in sport, including athletes and the staff of athletic programs, who may be at risk of exposure to human blood. The Committee on Sports Medicine and Fitness, a subcommittee of the Academy given the task of preparing the position statement, noted that the risks of infection were small, primarily because bleeding wounds are not particularly common and most blood-borne diseases require some access point to the blood in the non-infected person and require prolonged exposure to large amounts of infected blood. Nonetheless, a brief summary of the report’s recommendations are listed below:
- Athletes infected with HIV, Hepatitis B (HBV), and Hepatitis C (HCV) which are collectively the more serious blood-borne viruses, should be allowed to participate in all competitive sports.
- Infection status of athletes should remain confidential.
- Athletes should not be tested for infectious diseases.
- Physicians should council athletes infected with HIV, HBV, or HCV that they have a low risk of infecting others, but should take precautions against the spread of disease.
- Athletes and their parents should be made aware that the sport organization is operating under these recommendations.
- Clinicians and athletic staff should encourage all individuals related to sport get immunizations against HBV.
- All coaches and trainers must receive training in first aid and emergency care and in the prevention of transmission of blood-borne pathogens in the athletic setting.
- Coaches and athletic staff should educate athletes about the recommendations and precautions outlined in this position stand.
- While not required, it is a recommendation that athletic programs comply with Occupational Safety and Health Administration regulations of the region.
- Sports in which the risk of direct contact with an athlete’s blood is increased (e.g. wrestling or boxing) should take additional precautions to minimize the risk of pathogen transmission. Though the risk of transmission can never completely be eliminated, these precautions include:
- Existing cuts, abrasions, wounds, and other areas of broken skin should be covered and dressed appropriately in all participants in the athletic event. This includes, athletes, coaches, officials, trainers, etc. …
- Disposable, waterproof latex or vinyl gloves should be worn whenever addressing a wound or handling any blood-soiled clothing, bandages, or other materials.
- Athletes should be advised to report injuries and wounds in a timely fashion before or during competition.
- Contaminated equipment, clothing, and playing areas should be disinfected or replaced. Disinfection should include contact with an appropriate germicide or freshly-made bleach solution (1-part bleach in 10 parts water) for at least 30 seconds.
- Emergency care must not be delayed because protective equipment is not available.
- Breathing bags and sterile oral airways should be available at sporting events and be used preferably to mouth-to-mouth resuscitation.
- These precautions extend to equipment handlers, laundry personnel, and janitorial staff who must be trained in the proper handling and disposal of blood contaminated materials.
These guidelines are furthered by the National Athletic Trainers Association (www.nata.org) in their guidelines on how to prevent the spread of communicable and infectious diseases in secondary school sports:
- Immediately shower after each practice or competition.
- Wash all athletic clothing worn during practice or competition daily.
- Clean and disinfect gym bags and/or travel bags, if the athlete is carrying dirty workout gear home to be washed and then bringing clean gear back to school in the same bag (note: instead of disinfection, using disposable bags for practice laundry could be introduced).
- Wash athletic gear (such as knee or elbow pads) periodically and hang to dry.
- Clean and disinfect protective equipment such as helmets, shoulder pads, catcher’s equipment, and hockey goalie equipment on a regular basis.
- Do not share towels or personal hygiene products with others.
- All skin lesions should be covered before practice or competition to prevent risk of infection to the wound and transmission of illness to other participants; only skin infections that have been properly diagnosed and treated may be covered to allow participation of any kind.
- All new skin lesions occurring during practice or competition should be properly diagnosed and treated immediately.
- Playing fields should be inspected regularly for foreign objects and debris that could cause cuts or abrasions.
- Playing fields should be inspected regularly for animal droppings that could cause bacterial infections of cuts or abrasions.
- Athletic lockers should be sanitized between seasons.
- Rather than carpeting, locker or dressing rooms should have tile floors that can not only be cleaned, but also sanitized.
- Wrestling and gymnastics mats should be sanitized daily.
- Weight room equipment – including benches, bars, and handles – should be cleaned and sanitized daily.
—-
Functional Organs of the Immune System
The Lymph System
If you are asked to indicate the part of your body most responsible for glucose homeostasis, you might say the pancreas (because of its glucose regulatory hormones) or liver (because of its role in gluconeogenesis and secretion of glucose). Now think about what organs are important to the immune system. Likely, the first thing to come to mind is not an organ at all, but rather components of the immune system including inflammation, white blood cells, or antibodies. This is because the cells of the immune system can be found in nearly all regions of the human body. In many instances these locations correspond with sites of potential pathogen entry (e.g. tonsils at the entry to the throat), but this is not a requirement. The lymph system is actually made up of the lymphoid tissue, vessels, and lymph fluid. The word lymph is of Latin and Greek origin (lymph meaning water) and refers to a clear, transparent, sometimes yellowish fluid that circulates around the body in lymphatic vessels. Lymph contains primarily white blood cells, lipids and lipid-soluble vitamins, and other material collected from the extracellular space through-out the body. Lymph drains into the venous blood system at the thoracic and right lymphatic ducts. Lymphoid tissue is rich in cells named lymphocytes, but also contains accessory immune cells such as macrophages and reticular cells (a type of fibroblast). Lymphocytes and macrophages are described in more detail later in the chapter. The lymphoid system (see Figure 15-1) consists of the lymph vessels, the primary organ of the thymus gland, as well as the adenoids, tonsils, appendix, spleen (located at the upper left of the abdomen), lymph nodes (bundles of lymphoid tissue located along the lymphatic vessels with concentrations in the neck, armpits, abdomen, and groin), and Peyer’s patches (bundles of lymphoid tissue located along portions of the intestines). The thymus is located in front of the aorta and behind the sternum and varies considerably in size, such that in newborns and children, the thymus is relatively large, but progressively decreases in size into adulthood. The secondary lymphoid tissues are located close to anatomical sites in close contact with the external environment (e.g. throat and intestines) where they can best intercept pathogens before they enter the blood.
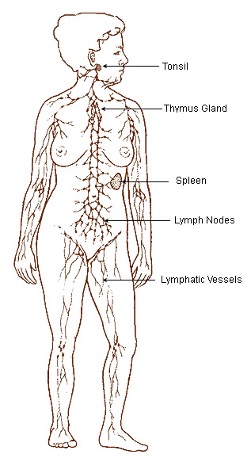
Bone Marrow
Lymphocytes typically mature in the thymus and other lymph tissues but originate in the bone marrow, the primary site of undifferentiated precursor cells called bone marrow stem cells (BMSC). As such, the bone marrow is considered one of the primary anatomical sites of the immune system. It is for this reason there is such concern with diseases that affect the normal functioning of BMSCs (e.g. leukemia, a type of cancer that causes excessive production of white blood cells) or therapies that may destroy the bone marrow, such as radiation or chemotherapy used to fight some cancers.
The Blood and its Constituents
In addition to the lymph, the blood, blood cells, and the vascular system are important components of the immune system. Human blood is composed of the plasma, red blood cells, platelets, and white blood cells. The plasma makes up approximately 60% of whole blood, is predominantly water-based and acts as a carrier for all blood-borne substances. As you have learned in previous chapters, the blood vessels provide the conduits through which the blood, and hence blood cells, travel. White and red blood cells are products of haematopoiesis (from the Greek: haima for blood and poiein to make) or the making of blood cells in the bone marrow. The red blood cells (erythrocytes) contain haemoglobin and thereby carry oxygen throughout the body. The platelets (thrombocytes) are cell fragments important to the clotting process whereby open wounds in the vasculature are sealed to prevent blood loss and maintain the integrity of the first line of defense against invading pathogens. The white blood cells or leukocytes compose the buffy coat or middle layer of centrifuged whole blood and are the cells most directly involved in immune system action. For this reason, standard blood tests examine the white blood cell count and use this measurement as an indication of immune health, infection, or stress. The typical complete blood count (CBC) is presented in Table 15-2. Standard tests of health typically include some portion of the CBC.
|
|
||||
|
Cell Type |
Count (K/uL) |
% of Whole Blood |
% of WBC Count |
Time in the blood |
|---|---|---|---|---|
|
RBC (erythrocytes) |
|
|
|
Months |
|
male |
4700-6100 K/µL |
41-50% |
|
|
|
Female |
4200-5400 K/µL |
36-45% |
|
|
|
Platelets (thrombocytes) |
147-347 K/µL |
|
|
days/weeks |
|
WBC (leukocytes) |
3.3-8.7 K/µL |
~1% |
|
|
|
Granulocytes |
|
|
|
|
|
Neutrophils |
|
|
54-62% |
1 day |
|
Eosinophils |
|
|
1-6% |
d |
|
Basophils |
|
|
<1% |
days |
|
Agranulocytes |
|
|
|
|
|
Monocytes |
|
|
2-8% |
days |
|
Lymphocytes |
|
|
25-33% |
years |
Note: these are typical ranges only and you should be aware that they may vary depending on the lab in which a blood test is done. Normal counts are thousands of cells per cubic millimeter or microliter of blood (KµL-1).
Platelets Mediate Clotting
The primary function of the platelets is to prevent blood loss from the vasculature through the coordinated actions of coagulation and clotting (i.e. the grouping together of platelets into thick globs that seal blood vessel breaches). This role can be both external (think about the scabs you see on a young soccer player’s knees) or internal (you might have heard about the danger of blood clots that are only detectable with more invasive devices). As noted above, the platelets or thrombocytes (Greek: thrombos meaning clot and cyte meaning cell) are actually cell fragments that originate from megakaryocytes formed in the bone marrow. The importance of the thrombocytes to normal physiological function, especially during athletic activity, cannot be overstated. For example, in a survey of 66 NCAA Division I team physicians, 64% noted that they would not allow an athlete with severe haemophilia, a genetic disease that prevents the clotting process, to participate in non-contact sports. An overwhelming majority (all of the physicians who responded to that question, ~83%) would not allow haemophiliacs to participate in contact sports (Fiala et al. 2003). While the dangers of a disease such as haemophilia are not directly immune related, not being able to seal any breaches to the vascular system could lead to life-threatening losses of blood.
Normal Flora
It is likely that you have heard the claim that the majority of the cells in your body are not human. If not, consider this: the human body is composed of approximately 1013 cells. However, in nearly every region of our bodies that are exposed to the external environment including the skin, oral and nasal cavities, gastrointestinal and urogenital tracts, vagina, and upper respiratory tract, we are normally colonized by a variety of micro-organisms that benefit our health. In fact, there are approximately 1012 bacteria that live on the skin and a whopping 1014 bacteria (over 400 separate species) that colonize the large intestine (Berg 1996). These bacteria provide several beneficial functions such as aiding in the motility of foodstuffs through the intestines, maintaining the integrity of mucosal tracts, and promoting the acidity of the vaginal tract through metabolism. With respect to the immune system, normal flora typically out-compete pathogenic bacteria for specific niches in the body. To this effect, they produce and release antimicrobial enzymes and substances into their local environment which can disrupt and destroy foreign bacteria. In healthy individuals, the normal gut flora periodically translocate across the intestinal barrier but are quickly neutralized by the host immune system. This may serve to prime the host immune system for quicker action (Berg 1996) and is important to normal immune system maturation (Marshall 1998).
When the immune system is weakened or compromised, or when the normal flora enter the body in excessive amounts through breaks in the epithelial barrier, an immune response may be invoked. Further, when the normal gut flora are eliminated, such as due to antibiotic drug treatments, unprotected niches may be opened up to more harmful pathogenic microorganisms. Interestingly, it has been hypothesized that during strenuous exercise when blood flow is redirected away from non-essential organs such as the intestines, the gut may experience transient periods of ischaemia (i.e. inadequate blood flow, and hence inadequate oxygen, nutrient, and waste removal/supply to an area). Because of this ischaemia, the gut mucosal and epithelial barriers, and the gut-associated lymph tissue, may be compromised allowing bacteria and their toxins into the body. Consequently, this may be part of the gastrointestinal discomfort and whole body inflammation (a standard immune response to infection) experienced during exercise (Marshall 1998; Pedersen et al. 1998). Evidence to support this hypothesis will be presented later in the chapter.
Immune Signaling Molecules
While foreign pathogens typically present their own danger signals, there are 3 types of chemical messengers that have a multitude of functions around the body including vital signaling and propagation of both a healthy and dysfunctional immune system. These chemical messengers fall within the ever-growing families of cytokines, eicosanoids, and chemokines.
The Cytokines
The cytokines (Greek: cyto for cell and kinos meaning movement) are important for intercellular communication and trafficking. While cytokines have predominantly been associated with inflammation and the immune system, they have recently been implicated in other physiological processes including exercise (described later in this chapter). The cytokines are predominantly glycoprotein chemical messengers, originally termed monokines and lymphokines because of their association with monocytes and lymphocytes, respectively. When it was determined that other cell types could synthesize and release these molecules, they were given the more inclusive name, cytokines. Now it is clear that nearly all cell types can secrete cytokines, but their roles in inflammation and immune cell signaling remain predominant. The individual molecules making up the cytokines do not have a distinct nomenclature and consequently many were named according to their observed function (e.g. tissue necrosis factor alpha, TNFα), their cell targets, and/or their origins (e.g. interleukin-1, IL-1). This nomenclature may be confusing given the multitude of roles observed today for any given cytokine, many of which are portions of complex signaling networks that may be redundant, synergistic, or even counteracting. While the cytokines can be grouped according to structural similarity based on amino acid sequence homology (Sprang and Fernando Bazan 1993), there are 7 functional families (Liles and van Voorhis 1995) of most major cytokines, including: the haematopoietic growth factors – which stimulate the proliferation and differentiation of blood cells.
- interferons – primarily anti-viral (“interfering” with the ability of viruses to replicate themselves).
- lymphokines – primarily produced by T-lymphocytes, these cytokines activate and signal other immune cells.
- monokines – cytokines produced by monocytes and macrophages.
- chemokines – involved in the chemical attraction and activation of immune cells (chemotaxis) (described below).
- other cytokines – in which roles cannot be distinctly defined.
Though not exhaustive, Table 15-3 lists many of the cytokines important to immune function and gives their primary roles in inflammation and immunity. The cytokines are primarily involved in local (autocrine and paracrine) intercellular communication and invoke responses by binding to specific membrane spanning receptors on target cells. During immune system activation and inflammation, cytokines levels can increase dramatically around the site of infection but also in the blood. It is important to note that while the plasma concentration of most cytokines is very low or undetectable, the observation of cytokines in the blood under some non-infection related conditions implicates them in more systemic or whole body responses. Naturally, from a diagnostic standpoint, cytokine profile blood tests can be used to characterize the state of the body with respect to stress or inflammation.
|
|
Pro-inflammatory |
Anti-inflammatory |
Involved in Acute Phase Response |
Modified by Exercise |
||
|---|---|---|---|---|---|---|
|
|
Cell-Mediated Immunity |
Humoral Immunity |
Allergic Immunity |
|
|
|
|
Interleukin (IL) |
|
|
|
|
|
|
|
IL-1 |
• |
• |
|
|
• |
|
|
IL-2 |
• |
• |
|
|
|
|
|
IL-3 |
|
|
• |
|
|
|
|
IL-4 |
• |
• |
• |
• |
|
|
|
IL-5 |
|
• |
• |
• |
|
|
|
IL-6 |
• |
• |
|
• |
• |
• |
|
IL-7 |
• |
|
|
|
|
|
|
IL-8 |
|
|
|
|
• |
• |
|
IL-9 |
|
|
• |
|
|
|
|
IL-10 |
• |
• |
|
• |
|
• |
|
IL-11 |
• |
|
|
|
|
|
|
IL-12 |
• |
• |
|
|
|
|
|
IL-13 |
|
• |
• |
• |
|
|
|
IL-15 |
• |
• |
|
|
|
|
|
IL-16 |
• |
|
|
|
|
|
|
IL-17 |
• |
|
|
|
|
|
|
IL-18 |
• |
|
|
|
|
|
|
IL-19 |
|
|
|
• |
|
|
|
IL-20 |
|
|
|
• |
|
|
|
IL-21 |
• |
• |
|
|
|
|
|
IL-22 |
|
|
|
• |
|
|
|
IL-23 |
• |
|
|
|
|
|
|
IL-24 |
|
|
|
• |
|
|
|
IL-25 |
|
• |
• |
|
|
|
|
IL-26 |
|
|
|
• |
|
|
|
IL-1ra |
|
|
|
• |
|
• |
|
Tissue Necrosis Factor (TNF) |
|
|
|
|
|
|
|
TNFα |
• |
|
|
|
• |
• |
|
TNFβ |
• |
|
|
|
|
|
|
Interferons (IFN) |
|
|
|
|
|
|
|
IFNα |
• |
|
|
|
|
|
|
IFNβ |
• |
|
|
|
|
|
|
IFNγ |
• |
|
• |
|
• |
|
|
Transforming Growth Factor (TGFβ) |
|
• |
|
|
• |
|
|
Stem Cell Factor (SCF) |
|
|
• |
|
|
|
|
Colony Stimulating Factor (CSF) |
|
|
• |
|
• |
|
While all of the cytokines play important roles in immune function, the chemokines are sometimes considered their own class of molecules. Chemokines (Greek meaning “chemical” and “movement”) are usually small cytokines that are important because they cause immune cell migration towards the area of highest chemokine concentration, a process called chemotaxis. Chemotaxis is the primary chemical event that coordinates the congregation of immune cells at the site of infection. Some of the interleukins are chemokines, but many chemokines possess unique names that can be grouped by their structural identities.
Physical exercise is a multifaceted stress, and not surprisingly, is a potent modifier of the cytokines that often mimics the response to other stresses. In the plasma of exercising individuals, the cytokines and chemokines are elevated in an exercise dose-dependent manner. These changes can have both positive and negative effects as we will see later in this chapter.
Some Cytokines Activate the Acute Phase Response
The local response to a tissue injury or infection by pathogen involves the release of cytokines in a coordinated sequence of events. However, during this acute phase of infection or inflammation, a host of other proteins (called acute phase proteins) can be observed to increase (>25%) in the plasma, thereby regulating a more global immune response. Some cytokines, produced by early responding immune cells such as macrophages, are highly associated with activation of the acute phase response. They are noted in Table 15-3. These cytokines can induce the liver, the primary site of proteins, to increase hepatocyte metabolism and initiate secretion of proteins. Interestingly, a cytokine profile response similar to that seen with infection and other trauma such as burns or surgery is observed after intense exercise or after eccentric contractions. This is part of the evidence of the power exercise has on modification of the immune system. One notable protein is called C-reactive protein (CRP). CRP may be both pro-inflammatory and anti-inflammatory but appears to be a key marker of pathophysiologic inflammation, hence its use as a diagnostic tool of disease severity, including heart disease. Other proteins play roles in activation of the complement system, (see below) coating of bacterial walls to target them for immune reaction in a process called opsonisation, and wound healing/tissue repair. Note that the proteins constitute one part of the acute phase response, which also includes more observable physiological changes such as fever and fatigue.
The Eicosanoids
The eicosanoids are a large group of fatty acid molecules that, like the cytokines play important roles in local cell-to-cell communication and, in particular, inflammation. In mammals, eicosanoids are primarily synthesized from the naturally occurring arachidonic acid, which itself is a product of enzymatic modification of released cell membrane phospholipids. The enzyme responsible for the production of arachidonic acid is called phospholipase A2. Phospholipase A2 appears to be the rate limiting step in eicosanoid synthesis and physiological expression given that eicosanoids can diffuse through the cell membrane. Arachidonic acid is the precursor to all 3 families of the functionally active eicosanoids: the prostaglandins, thromboxanes and leuokotrienes. These names stem from their respective origins of first discovery. The prostaglandins were first observed in the seminal fluid (originating from the prostate gland), the thromboxanes were platelet derived factors that had key roles in thrombosis (clot formation) and the leukotrienes were initially characterized as leukocyte derived signaling molecules. The prostaglandins and thromboxanes are collectively termed prostanoids and synthesized subsequent to the enzymatic modification of free arachidonic acid by the enzyme cyclooxygenase. If you have ever taken the drug Aspirin™ to reduce the pain associated with an injury, you have targeted this eicosanoid synthesis pathway. Cyclooxygenase is the primary target of Aspirin™ and related non-steroidal anti-inflammatory drugs (NSAIDs) which cause inhibition of the enzyme. Ibuprofen, another common NSAID, reversibly competes for the arachidonic acid binding site in cyclooxygenase, thereby accomplishing the same goal: inhibition of pro-inflammatory prostanoid synthesis. In order to generate leukotrienes, free arachidonic acid is modified by the enzyme lipoxygenase. The nomenclature for the eicosanoids is more unified than the cytokines and this can be observed in Table 15-4. The eicosanoids are extremely short-lived with half-lives in the seconds to minutes and have predominantly autocrine and paracrine roles. Consequently, they are expressed in extremely low plasma concentrations (10-9 M) in healthy individuals. Much like the cytokines, however, physical exercise can increase the expression of many eicosanoids that have important physiological functions such as vasoconstriction/dilation (sciencedirect.com). The eicosanoids primarily act on membrane spanning g-protein coupled receptors and have target cells from nearly every organ in the human body. Like the cytokines, the eicosanoids perform a multitude of diverse functions, making classification by this route difficult. For example, some eicosanoids may be associated with bronchodilation whereas others are highly correlated with bronchoconstriction. As a result of exercise, a change in the amount of broncho-constricting eicosanoids released from immune cells may be a factor in exercise-induced bronchoconstriction and asthma (Hallstrand et al. 2005). Nonetheless, for the purposes of this chapter, the important functions of the eicosanoids lie in their ability to influence local immune cell congregation, inflammation.
|
Eicosanoid |
|
Major Biological Activities |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Vasodilation |
Vasoconstriction |
Vascular Permeability |
Bronchoconstriction |
Platelet Aggregation |
Leukocyte Aggregation |
Lymphocyte Proliferation |
Induces Cytokine Secretion |
||
|
Family |
Name |
Major Site(s) of Synthesis |
||||||||
|
Prostaglandins and Prostacyclins (PG) |
|
|
|
|
|
|
|
|
|
|
|
|
PGD2 |
mast cells |
↑ |
|
|
|
↓ |
↓ |
↓ |
↑ |
|
PGE2 |
kidney, spleen, heart |
↑ |
|
|
|
↑ |
|
↓ |
↑ |
|
|
PGF2α |
kidney, spleen, heart |
|
↑ |
|
↑ |
|
|
|
|
|
|
PGH2 |
many sites |
|
↑ |
|
|
↑ |
|
|
|
|
|
PGI2 |
heart, vascular endothelial cells |
↑ |
|
|
|
↑ |
↑ |
↓ |
|
|
|
Thromboxanes (TX) |
|
|
|
|
|
|
|
|
|
|
|
|
TXA2 |
platelets |
|
↑ |
|
↑ |
↑ |
|
↑ |
|
|
TXB2 |
platelets |
|
↑ |
|
|
|
|
|
|
|
|
Leukotrienes (LT) |
|
|
|
|
|
|
|
|
|
|
|
|
LTB4 |
immune cells |
|
|
↑ |
|
|
↑ |
↑ |
↑ |
|
LTC4 |
immune cells |
↑ |
|
↑ |
↑ |
|
|
|
↑ |
|
|
LTD4 |
immune cells |
↑ |
|
↑ |
↑ |
|
|
|
↑ |
|
|
LTE4 |
mast cells and basophils |
↑ |
|
|
↑ |
|
|
|
|
|
Complement Proteins
Complement refers to a large number of plasma proteins and cell surface proteins that function as enzymes, binding proteins and immune cell activating molecules. Complement proteins recognize some pathogens and infected cells and bind to them. This binding typically results in bacterial destruction by one of two methods:
- Several complement proteins congregate on the infected cell forming a membrane attack complex that forms channels or pores in the infected cell’s membrane leading to cell death.
- Complement coated cells attract immune cells to target the infected cell for destruction.
Complement proteins typically circulate in the blood plasma in inactive form but are activated by pathogenic material. The cascade of component activation and congregation occurs in an ordered fashion to accomplish the actions listed above. The proteins are given the names C1, C2, C3, etc. which represents the order of activation.
Cells of the Immune System
All Immune Cells are Leukocytes
The leukocytes (leukos, Greek for white), or white blood cells, is the family name of nearly all of the cells of the immune system. They are so named because of their distinctly colourless or white appearance relative to the erythrocytes (red blood cells) found in the blood. Leukocytes are formed by the process of haematopoiesis in the bone marrow from a common blood cell precursor. Figure 15-2 shows the various lineages of the common white blood cell lines. It is also important to note that while they are found predominantly in the blood, this is primarily a vehicle of a transport for the leukocytes. In fact, many of the functions of the leukocytes occur outside of the blood vessels and are due to a special ability of the white blood cells to exit blood vessels through small pores in the capillary endothelium. The leukocytes are not, however, a homogeneous mix of cells and consequently their nomenclature requires further characterization. Leukocytes are generally classed by the nuclear and granular expression in each cell. Leukocytes may either be mononuclear containing a single round nucleus or polymorphonuclear containing a varied shape nucleus that may appear divided. Granules are small secretory vesicles located in the cell cytoplasm that contain chemical molecules (e.g. lysing enzymes and cytokines) that are released upon stimulation in a process referred to as degranulation. Some leukocytes have many granules and are consequently considered granulocytes, while other leukocytes contain few to no granules and are therefore considered agranulocytes. While each cell type can usually be further classified as belonging to either the innate or acquired immune systems, their coordinated actions are a requirement of proper immune health.
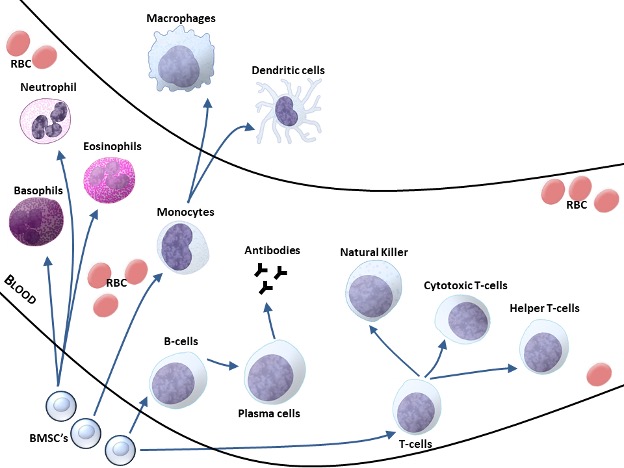
The Granulocytes
The granulocytes are so named because of the appearance of granules (small particles that contain various enzymes, proteins, and signaling molecules) that pock mark the cytoplasm of this type of leukocyte. It is the pattern of histological staining of the granules with acidic and basic dyes that gives each subsequent class of granulocyte its name. The neutrophils, the most abundant of the leukocytes, exhibit neutral pink staining of the cytoplasm while the eosinophils (acid-liking) exhibit brick red staining and the basophils (basic-liking) exhibit a dark blue colour after hematoxylin (blue) and eosin (red) staining. All of the granulocytes can undergo degranulation in which the contents of the granules are released to the surrounding extracellular area.
Neutrophils
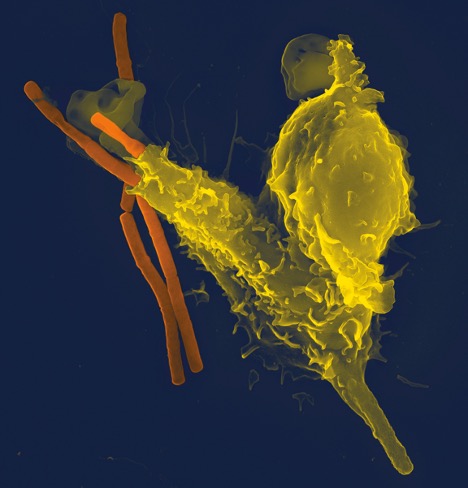
In the innate defense against pathogens, tissue embedded neutrophils [also called polymorphonuclear neutrophils (PMN) or polys for short] are typically the first responders to react after detection of certain chemoattractants. These chemoattractants can include the membrane proteins of certain bacteria, bacterial toxins, or host cellular proteins such as cytokines, chemokines, and eicosanoids or inorganic ions such as calcium. Nonetheless, these signals typically denote foreign invasion or the disruption of host cellular integrity. Movement to the areas of high chemical attractant by neutrophils is known as chemotaxis (chemo-, chemical and taxis, Greek for arrangement or order) and allows many neutrophils to amass at a site of infection. If the early arriving neutrophils are insufficient to defend against the attack, circulating neutrophils can exit the blood vessels through tiny gaps by a process termed diapedesis (pedesis is Greek for movement). After congregating at the site of infection by chemotaxis, the neutrophils defend against foreign bodies by adhering to, engulfing, and ingesting the pathogen (e.g. shown in Figure 15-3). This process is termed phagocytosis (phagein is Greek for eat) and is common to the other granulocytes as well as monocytes and dendritic cells, consequently providing another grouping for cells with this specialized ability as phagocytes. Phagocytosis results in the containment of foreign matter inside a packaged vacuole which can fuse with lysosomes containing degrading enzymes inside the phagocyte, leading to pathogen digestion. Activated neutrophils undergoing degranulation can release proteases that aid in the digestion of foreign particles or other chemoattractants such as chemokines, eicosanoids, and cytokines that signal other immune cells to the area (Wright et al. 2010). The critical part of this entire process is that the neutrophils recognize the pathogen based on a standard set of chemoattractants. If the pathogen does not possess a known attractant, it can evade destruction – often providing enough time for reproduction and spread of the infection.
Eosinophils
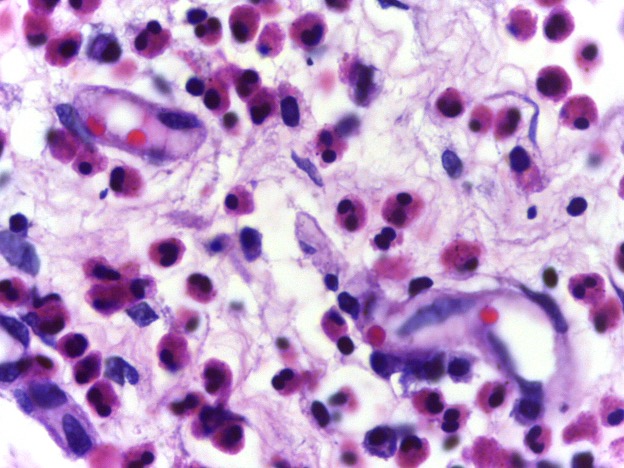
Eosinophils possess the ability to detect and destroy foreign invaders through phagocytosis; however, they circulate in much smaller concentrations than the neutrophils but last several days longer. In addition to phagocytosis, activated eosinophils can release or secrete a host of chemoattractants as well as granule proteins that can degrade surrounding biological material (Kita 1996). Another important difference between the eosinophils and neutrophils is that the eosinophils are not typically detected in the esophagus, lungs, skin, and some internal organs whereas the neutrophils can be found in the blood and tissues all over the body. In fact, when eosinophils are detected in these locations, it is usually a sign of disease. For example, in individuals who experience exercise-induced asthma, a higher concentration of eosinophils are detected in the lungs. This will be discussed later in this chapter. Nonetheless, because eosinophils and the basophils described below typically mediate allergic responses, they can be grouped into an arm of the immune system known as allergic immunity. See Figure 15-4 for a micrograph image of eosinophils.
Basophils and Mast Cells
In contrast to the neutrophils and eosinophils, basophils travel in the circulation but are relatively rare during normal circumstances. Basophils are very similar to mast cells (they are granulocytes that originate from the same precursor cell) but the latter typically congregate in the connective tissue of the skin, lungs, and gastrointestinal tract where they can stop and neutralize pathogens that breach these areas. These granulocytes bridge actions in both the innate and acquired immune systems, either by interaction with antibodies produced by T-lymphocytes of the acquired immune system (see below) or through direct detection of some bacteria and pathogens. They can release histamine, heparin, proteases, cytokines, and eicosanoids that are important mediators of inflammation and the immune response, but also can change blood vessel tone and smooth muscle contraction in the gut and vasculature (Galli 2000). Interestingly, Basophils and mast cells are often considered the “bad guys” in the immune response because of their roles in allergic disorders including anaphylaxis and asthma in which the immune system is considered hypersensitive. In this regard, histamine is one of the key mediators of allergic reactions and if you or anyone you know typically suffers from allergies, you will be familiar with the role of anti-histamine drugs to combat a hypersensitive immune system.
Monocytes (Macrophages and Dendritic Cells)
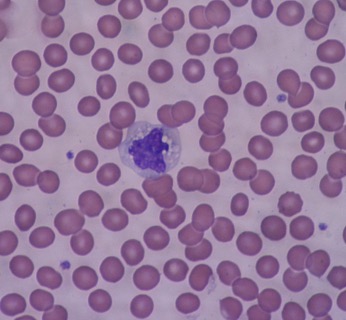
Monocytes, like all leukocytes, originate in the bone marrow, but are more similar to the lymphocytes as they are mononucleated. Monocytes can be released into the circulation where they can spend hours to days before differentiating into macrophages or dendritic cells (Figure 15-5). In most body tissues this replenishes basal macrophage numbers but can also increase the number of WBC in response to pro-inflammatory immune signals. Circulating monocytes can be classified based on the expression of certain antigenic markers standardly named cluster of differentiation (CD) markers. Most monocytes specifically express large amounts of CD14 (therefore they are considered CD14+) but it is the relative expression of antigenic proteins, chemokine receptors, and the local extracellular milieu that determine whether monocytes will differentiate into macrophages or dendritic cells. Further, both macrophages and dendritic cells are heterogeneous groups of cells whose phenotype and function is dependent on their local environment (Gordon and Taylor 2005).
Macrophages
Once differentiated, macrophages contribute to host innate immune responses by engulfing pathogens (phagocytosis) but also aid in healthy tissue maintenance and repair by clearing away dead cells and cellular material. For example, novel and/or intense exercise, primarily resistance or eccentric type training, is associated with skeletal muscle damage on both micro and macro scales (Smith 1991), leading to the consequent symptoms of delayed onset muscle soreness (DOMS). Clearing of these damaged myocytes (muscle cells) and other cellular debris is primarily accomplished by local macrophages and is an important component of muscle recovery and repair (Butterfield et al. 2006). This will be discussed further later in this chapter. Mature macrophages can be fixed to certain locations or mobile throughout specific tissues, actively patrolling for dead cells or foreign invaders. While smaller in number, the phagocytic activity of macrophages is much greater than the circulating neutrophils. Further, because these immune cells function without the aid of antibodies, this type of immunity is known as cell-mediated immunity. The granulocytes, macrophages, natural killer cells, and cytotoxic T cells (described below) all play a part in cell-mediated immunity.
Dendritic Cells
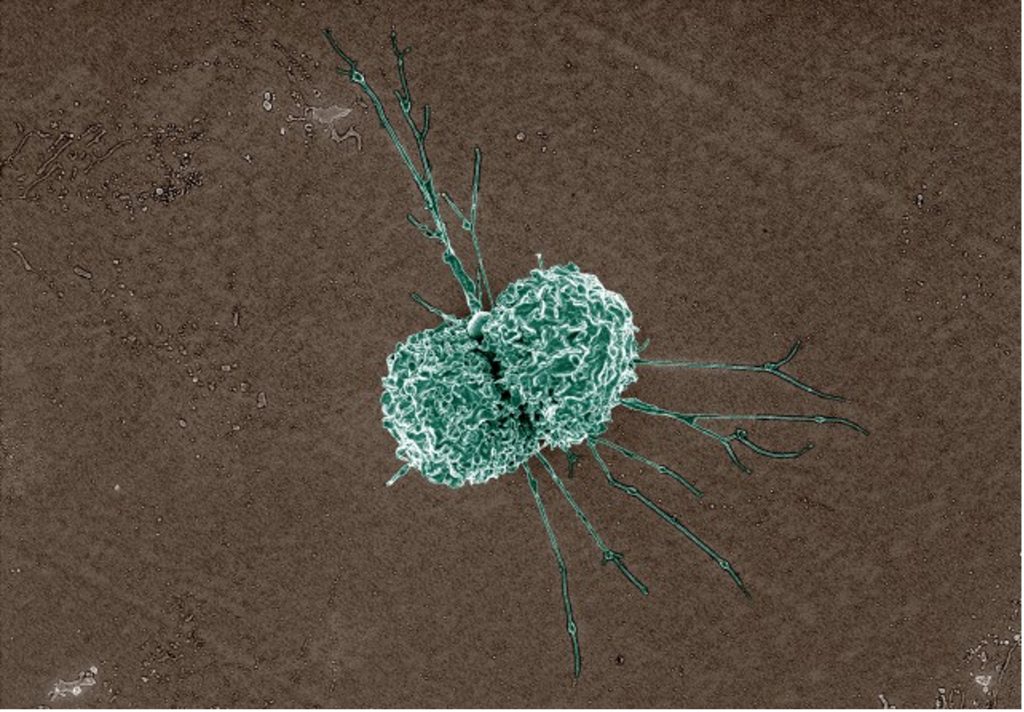
Dendritic cells are so named because of their large cellular extensions much like the dendrites of nerve cells (Figure 15-6). When monocytes differentiate into dendritic cells, they do not typically remain at the site of tissue injury, inflammation, or infection. Dendritic cells can ingest (phagocytose) foreign and host cellular material but differ from macrophages in that they can re-enter the circulation and migrate to lymph nodes where they act as antigen presenting cells (APCs) for T-lymphocytes of the acquired immune system. Antigen presenting cells digest foreign material and then express parts of the digested cells on their surface, typically proteins. These proteins (“antigens”) can then be “presented” in a way that cells of the acquire d immune system can recognize them and become activated. Macrophages and other cells can act as APCs, but do not circulate back to the lymph organs for cell priming. As an unfortunate yet ingenious method of infection, this is the method by which the HIV virus infects and consequently is able to disable the cells of the immune system.
Lymphocytes
The lymphocytes are mononuclear cells that typically lack the granular expression of the immune cells previously mentioned. They include the B-lymphocytes and T-lymphocytes which play large roles in the ability of the immune system to “remember” certain pathogens and respond in greater amount after a second exposure.
B-Lymphocytes
The B-lymphocytes (or B cells) were so named because they were observed to originate from the Bursa midura in birds and, coincidentally, the bone marrow in mammals. Recall that all leukocytes originate in the bone marrow so this is not really a distinguishing feature. B-cells are specialized lymphocytes that possess antibodies on their cell surface and can produce and secrete antibodies into the blood. When B-cells are activated due to antigen binding, they begin to divide and separate into 2 cell types:
- plasma cells that produce antibodies.
- B memory cells that are important to the immune system‘s ability to “remember” specific antigens.
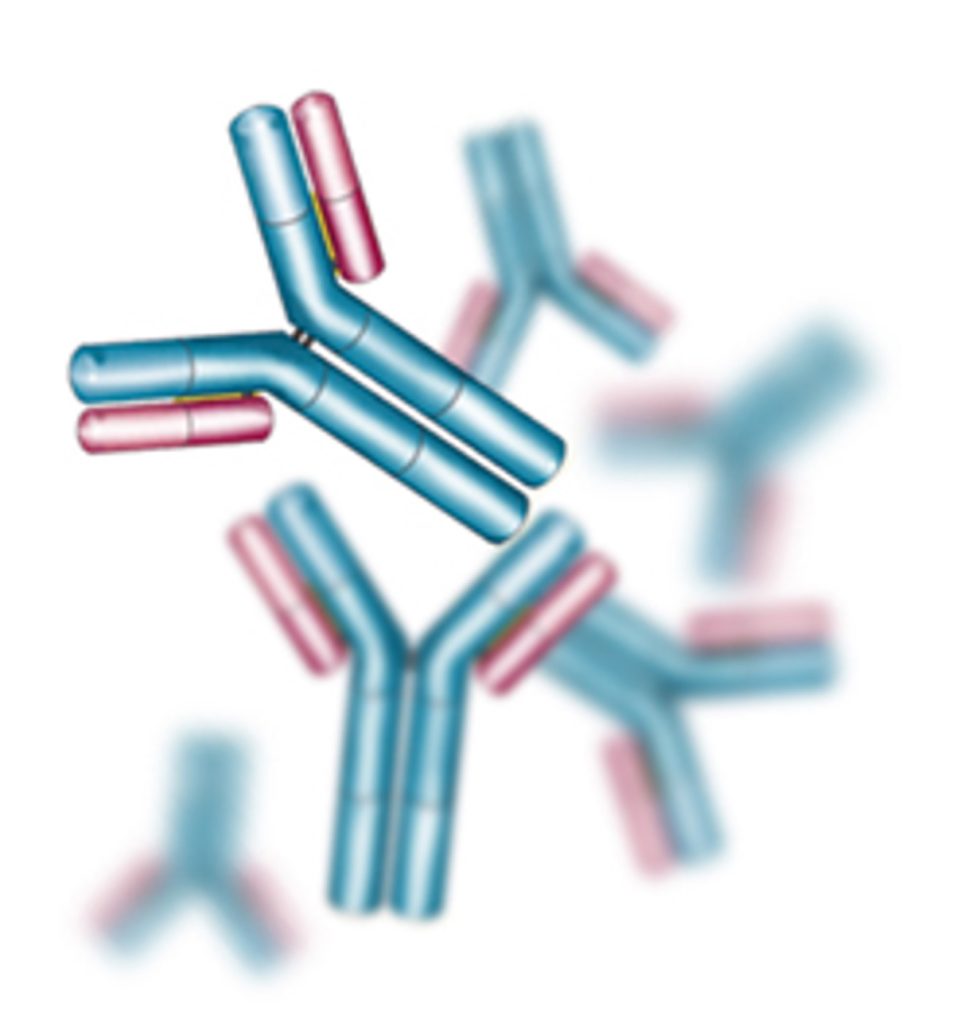
Antibodies, also called immunoglobins (Ig), are Y-shaped proteins (Figure 15.7) that possess antigen specific binding sites and can be used to target and flag pathogenic bacteria and other material. Because antibodies are released into the blood and around tissues, the B cells give rise to what is known as antibody mediated or humoral (“in the blood or body fluid”) immunity. The ability of B cells to generate antibodies against specific antigens and store this information is a key component of the acquired immune system. However, once produced, some antibodies (IgM, IgA) are involved in immediate responses to pathogen. For example IgA and IgM antibodies form part of the mucosal and salivary defenses in the linings of the gut, urogenital tract, respiratory tract, nose, and mouth where they assist in pathogen antigen recognition, aggregation, and destruction (Bouvet and Fischetti 1999; Gleeson and Pyne 2000)
—
Highlight Feature: Tinea Pedis (Athlete’s Foot)
Tinea Pedis is a non-life threatening fungal infection caused by dermatophytes (fungi that require keratin for growth) infection (Crawford 2009). This infection is more common in individuals who partake in sports, hence its more common name, athlete’s foot. It does not appear that its prevalence is higher in any particular type of sport, but rather it has been reported that athletes of all types show almost double the prevalence as the general population (Field and Adams 2008). The feet provide a favourable location for dermatophytes to thrive. This is because there are few host anti-fungal defenses in the feet. Also, in particular to the athlete, there is a greater chance that the feet:
- may be wet or damp due to sweating;
- are occluded by athletic footwear increasing heat and anaerobe favouring conditions;
- may be damaged due to athletic activity; and
- may come into contact with the fungi in communal showers and change rooms.
Athlete’s foot is highly infectious and scales from infected skin can remain infectious for several months. It is characterised by itching, flaking, and fissuring of the skin. It may manifest in three ways: the skin between the toes may appear macerated (white) and soggy; the soles of the feet may become dry and scaly; and the skin all over the foot may become red, and vesicular eruptions may appear (Crawford 2009).
The dermatophytes responsible for athlete’s foot are primarily of the type, Trichophyton rubrum and Trichophyton mentagrophytes, and with few exceptions, this appears to be similar in athletes although symptoms may be more robust (Auger et al. 1993). The general population may often come in contact with the fungi that cause athlete’s foot, however, it may not be able to manifest itself unless the immune system is weakened. In the case of the athlete, a depressed immune system as a result of high intensity high volume training can further increase the risk of fungal infection (Brenner et al. 1994).
While athlete’s foot is non-life threatening, it is important to treat the infection and prevent the possibility of it becoming systemic through more invasive cuts or abrasions. As presented in this chapter, once an infection becomes systemic (in the blood), other host defenses are called into play, presenting a more significant challenge for the individual. Athlete’s foot, like many superficial infections, can be successfully treated with topical creams or powders containing anti-fungal and anti-bacterial agents under most conditions, with the addition of corticosteroids when more severe.
Prevention includes both personal (changing socks, allowing athletic footwear to dry out, the use of preventative powders) and environmental (proper sanitation of communal areas) methods.
|
Immunoglobin (Ig) |
Typically Observed |
% of Total Body Ig |
Function |
|---|---|---|---|
|
IgG |
blood + extracellular fluid |
75-80% |
secondary immune response and acquired immunity; activates complement system
|
|
IgA |
nose, mouth, respiratory, urogenital, reproductive, and digestive tracts in external secretions (breast milk, gastrointestinal mucus, saliva, tears, etc.)
|
10-15% |
disable pathogen before internal entry; consequently primary immune defense and innate immunity; activates complement system |
|
IgM |
B cell membrane + extracellular fluid (lymph, blood) |
5-10% |
activate the complement system and therefore, are involved in primary immune responses
|
|
IgE |
extracellular fluid (lungs, skin, and mucous membranes)
|
~1% |
stimulate mast cells during allergic responses |
|
IgD |
primarily B-cell membrane |
<1% |
activate B-cells, basophils, and mast cells |
T-Lymphocytes
The T-lymphocytes (T cells) are so named because a large proportion of these cells are found in the thymus. Like all lymphocytes, T cells originate in the bone marrow but migrate to the thymus where they develop further and differentiate into subsets. These include Helper T (TH) cells, cytotoxic T (TC) cells, and the granular natural killer cells. In contrast to B cells and antibodies, T cells recognize infected cells and cannot bind free pathogen. In this regard they, along with the macrophages, are key components of cell-mediated immunity. Recognition of infected cells is accomplished by receptors on the T cells that interact with antigen in combination with major histocompatibility complexes (MHC). The MHCs are a family of cell surface proteins that hold portions of host cellular proteins or digested pathogen proteins in a conformation that can be recognized by T cells. While every nucleated cell in the body expresses related MHC proteins, non-immune cells typically only express MHC Class I (MHC-I) molecules on their cell surface. It is the totality of MHC-I- antigen expression that dictates whether host cells will be considered normal, abnormal, or infected. In contrast, “professional” APCs, such as the dendritic cells described above, typically express both MHC-I and MHC Class II (MHC-II) membrane proteins. The MHC-II proteins “present” antigen to T cells to induce immune action. You could think of the MHC and antigen presentation like a bar code on each cell. T cells can scan the barcode to determine if that cell “belongs” in the body. When infected or if the cell becomes abnormal, the T cells do not recognize the barcode and subsequently target that cell for destruction. This is a key method by which the immune system regulates dysfunctional cells in addition to infected cells. It should also be apparent to you that a dysfunctional immune system, such as one that cannot recognize normal from abnormal cells or one that is hyperactive and does not discriminate in the attack of cells, could become problematic.
Helper T Cells (CD4+)
Helper T (TH) cells are a major driving force of the immune system but they do not destroy cells. Instead, TH activate B cells and cytotoxic T cells after coming in contact with infected cells. In particular, TH cells are activated by APCs with MHC-II membrane proteins that “present” the TH cells with antigens specific to a processed pathogen. Simultaneously, APCs and other immune cells release cytokines, eicosanoids, and other signaling molecules that help to activate the TH cells, induce further TH cell differentiation, and stimulate the immune system. Activation can lead naïve TH cells (i.e. undifferentiated) to become one of 4 further subtypes, including:
- TH1 cells – that activate macrophages and natural killer cells (members of the innate immune system and cell-mediated immunity) and promote memory cell antibody (IgG) responses.
- TH2 cells – that predominantly increase B cell activity for antibody or humoral immunity, and eosinophil activity involved in allergic inflammation. They also suppress macrophages and cell-mediated immunity.
- TH17 cells – that recruit neutrophils and macrophages to infected tissues through the secretion of the cytokine Il-17.
- Regulatory T (Treg) cells – that suppress allergen induced T-cell activation as well as suppress mast cells, basophils, and eosinophils. Tregs and the cytokines they secrete are typically considered anti-inflammatory.
Each subset typically secretes a distinct set of cytokines and other immune signaling molecules that control immune action. For example, the cytokines released by the TH1 cells are termed TH1 cytokines while TH2 cells secrete TH2 cytokines. Consequently, TH1, TH2, or TH17 cytokines will be referred to later in the chapter but you should be aware that they ultimately result in the process listed above.
Cytotoxic T Cells (CD8+)
In contrast to TH cells, cytotoxic T (TC) cells are the “soldiers” of this lymphocyte population. TC cells can destroy infected cells when antigen is presented by MHC-I surface proteins. They accomplish this by releasing enzymes to disrupt and breach the cell membrane and induce programmed cell death (apoptosis). Apoptosis is a controlled method of destroying a cell, similar to the coordinated implosion of a building. In the case of a condemned building analogy, specialized crews go into the building to salvage materials that still hold value and then explosives are placed at designated sites to limit the amount of damage to neighbouring structures when the structure is destroyed and falls upon itself. In this regard, apoptosis is a method of pruning or eliminating specific abnormal cells.
Natural Killer Cells
Natural killer (NK cells) cells make up about 5 to 16 percent of the total lymphocyte population and are an important component of the immediate immune response to cancerous or pathogen-infected host cells. They are named “natural killer” cells because of their ability to destroy infected and malignant cells upon their first exposure to them (within hours) without presentation by typical pathogen related surface signals. NK cells possess similar actions to the cytotoxic T-cells; however, unlike the other lymphocytes, they express cytosolic granules in similar number to the granulocytes described earlier. Formed in the bone marrow and released into the circulation, NK cells can be recruited to different areas of the body by chemokine signals. Further, they can also migrate between body areas and perform their functions in several places. Like T cells, NK cells will interact with MHC-antigen expression on host cells, but they will also recognize when MHC-I- antigen molecules are altered or missing in stressed cells (Shi et al. 2011). This is an important component of the recognition of self and presents a method by which NK cells can remove cancerous cells.
The roles of NK and TC cells in addition to macrophages in monitoring cell activity is termed immunosurveillance because they patrol the body for cells that are abnormal, destroying and inhibiting the multiplication of cells that are deemed so. Consequently, an increase in the activity or amount of these cell types could potentially increase the removal of abnormal, often cancerous cells. Interestingly, with some forms of moderate intensity exercise basal levels of NK cell function, but likely not number, are elevated (Nieman et al. 1990; MacNeil and Hoffman-Goetz 1993). It has recently been suggested that this may be partly responsible for the reduction in some forms of cancer with regular exercise (Rogers et al. 2008).
The Immune System Employs Two Types of Defense
In order to successfully protect the body against pathogens that can take on almost endless identities, the immune system has evolved two separate but functionally linked branches. Nonspecific defenses are inherent to our biology and therefore passed on through genetic information. Specific defenses provide the ability to ward off and eliminate specific invading pathogens and are acquired or for which the host defenses adapt to the specific pathogen. Rather fittingly, these defense systems are referred to as innate and acquired or adaptive immunity, respectively, and vary in the speed and specificity of responses. Both types of immunity rely on their ability to:
- detect and identify foreign material
- signal and coordinate the actions of other immune cells
- contain, suppress, and/or destroy the pathogen
When invading pathogens make it past the skin, chemicals they secrete (known as toxins) can enter the blood or lymphatic vessels where they may then infect other areas of the body. Not surprisingly, the more global the infection, the more of a danger it is to the body. Consequently, the immune system attempts to limit this spread.
Innate Immunity: Defenses You Were Born With
Once past the physical and chemical barriers of the body, the second line of host defense against foreign pathogens involves the action of phagocytes. Remember that these are the devouring cells that Dr. Ilya Mechnikov observed gathering at a wound site following skin puncture and primarily include the neutrophils and macrophages upon first exposure. At the most basic level, these cells are responsible for recognizing, engulfing, and subsequently destroying foreign microbes. This is accomplished by a group of receptors on the cell membranes of phagocytes that recognize a large number of pathogen-associated molecular patterns (PAMPs) which may include biological material such as proteins, lipids, carbohydrates, and nucleic acids associated with the foreign agent. Of particular note is a type of PAMP called lipopolysaccharide (LPS) that is an endotoxin present on certain bacteria that induces massive immune responses and is used quite frequently in research studies to examine immune health. Not surprisingly, the immune cell receptors that recognize these PAMPs are called pathogen-associated pattern recognition receptors (PRR) that initiate an intra- (“inside”), and subsequently inter- (“between”), cellular signaling cascade leading to the secretion of cytokines, chemokines, and other anti-microbial or inflammatory substances. These events also lead to the congregation of immune cells at the site of infection. A superfamily of PRRs includes the toll-like receptors (TLR), so named because of their similar structure to a protein named Toll in fruit flies that is important to the immune response of those organisms. These receptors are found in all cells of the innate immune system but also in fat cells, liver cells, and muscle cells and are typically considered pro-inflammatory. s have only been characterized within the last two decades, but recent evidence suggests tha t exercise training can reduce the expression of TLRs in muscle and some leukocytes, thereby reducing the inflammatory process (see Research Box 15-1).
Research Box 15-1Heat Shock Proteins and Immunity: Potential Link to Exercise-Induced Anti-Tumor Immunity
The Canadian Cancer Society reports that 35% of all cancers can be prevented by being physically active. Further, the Center for Disease Control indicates that physical fitness along with reduced body weight is an important method of preventing several of the most common forms of cancer The mechanisms behind this relationship are unclear, but some of those mechanisms may include immune system modification. In particular, an increase in immunosurveillance and cytotoxic ability of the innate immune system may provide this link.
Recall that some immune cells can recognize abnormal host cells and target them for destruction. This process is known as immunosurveillance and is particularly accomplished by differentiated monocytes and NK cells. You have learned that toll-like receptors (TLR) are a type of pattern recognition receptor found on the cell membranes of leukocytes involved in innate immunity including neutrophils, monocytes (macrophages and dendritic cells), and NK cells. There are at least 10 isoforms of the TLRs with both independent and redundant functions (Takeda et al. 2003). In particular, TLR-4 is significant because it was the first pattern recognition receptor found to bind with lipopolysaccharide (LPS), a common bacterial toxin. Upon binding LPS or some other ligand, and together with other cell surface proteins such as CD14, TLR -4 signalling induces pro-inflammatory cytokine release from its host cell. Other TLR ligands that have recently gained interest are members of the heat shock protein (HSP) family.
HSPs are molecular chaperones that have several intra- and inter-cellular functions. Primarily these are:
- to prevent intracellular protein denaturation and aggregation
- to assist in the proper folding of newly synthesized and misfolded proteins
- to aid in the transport of proteins through various intracellular compartments
HSPs are also expressed on cell surface membranes and in the extracellular fluid and plasma where they may be free or bound to other peptides. These types of HSPs are known as extracellular HSPs (eHSPs). Some extracellular HSPs are ligands for TLR-2 an d TLR-4. These include the highly inducible 70 kDa HSP, HSP-70, and 60 kDa HSP, Hsp60. When HSP-70 binds to TLR-2 or TLR-4, they activate the immune cell possessing those receptors. Activation can cause increased activity of that cell and the release of TH1 or pro-inflammatory cytokines that initiate other immune responses (Asea et al. 2002).
NK cells possess TLR-2 and TLR-4 in sufficient quantities to be major targets of HSP-70 and Hsp60. Cell surface HSP-70 binds TLR-4 o n NK cells and enhances NK cell cytolytic activity in the presence of CD14. In this chapter, you learned that NK cells are integral in the removal of cancerous cells in the body. As such, an increase in NK cell cytolytic activity could potentially increase this ability.
Monocytes also interact with eHSP-70 including eHSP-70. This observation links eHSPs to antigen presentation of both foreign and endogenous cell material (think non-self versus self). Recall that circulating monocytes are precursors to macrophages and dendritic cells which possess the ability to phagocytose material and act as antigen presenting cells. Because HSP-70 binds with other proteins, antigen bound HSP-70 can interact with monocyte TLRs, be phagocytosed and then “presented” to lymphocytes of the adaptive immune system (Calderwood et al. 2008). Primarily, this results in activated cytotoxic T-cells that can then destroy the abnormal cells.
Given the role of HSPs in increased NK cells and T-cell cytolytic ability and antigen presentation, it is not surprising that some tumor derived HSPs are now included in anti-cancer vaccines and medications with successful outcomes. Further, it is likely that the use of different HSPs in future treatments will occur (Murshid et al. 2011).Intense exercise causes a significant increase in eHSPs, HSP-70 in particular (Febbraio et al. 2002; Lancaster et al. 2004). This extracellular HSP-70 appears to originate from the contracting skeletal muscle and brain during exhaustive exercise (Febbraio et al. 2002; Lancaster et al. 2004). The role of exercise-induced eHSP-70 is unknown, but given those functions listed above, it may increase immunosurveillance and anti-tumor immunity. Indeed, there is a concomitant increase in eHSP and NK cell associated HSP-70 after strenuous exercise (Horn et al. 2007). As such, exercise-induced eHSP, TLR-2 signalling, and immune function may provide one link between exercise and the reduced risk of cancer.
—
Interestingly, PRRs also recognize host-borne danger signals that are found when cells are damaged and release their intracellular contents. This should remind you that the innate immune system is not only to keep invaders out but also to clean up damaged cells and help in the repair of the body’s own tissues. Key components of the innate system include that:
- immunity is passed on from generation to generation and is present in individuals from birth (hence innate).
- the recognition and destruction is non-specific such that anything possessing the right type of PAMP will be targeted.
- this is a relatively fast acting response because all of the necessary components are present at the time of invasion.
Think about your body like a large factory with expensive equipment and materials in the warehouse and confidential documents in the offices. Thieves could steal your resources and competitors could steal your documents to make their products better. You fortify the factory with fences and gates and lock all of the doors at night. These are sufficient to keep out most unwanted visitors, but for enhanced protection you have also installed an alarm system that will alert you and the authorities if any entry point is breached and you have employed a night watchman who patrols the factory and offices. Now your factory is well guarded against all but the most sophisticated and/or overwhelming threats. An important component of this system is that you and your trusted employees are able to get into the factory. For this, you give them all pass keys that open most doors, and photos of your most trusted employees are memorized by the security guards. Think about the series of events that will occur if someone cuts through your outer fences and manages to pry open one of the windows. First of all, if your alarm system is working, a loud siren will sound and indicate to the central watchman where a breach has occurred. Simultaneously, this alarm will contact the local authorities, but it will be some time before they reach the factory. The sound and location of the alarm will cause nearby security guards to rush to the spot to investigate whether there has been an actual break-in. If they cannot handle the threat (say there are several burglars at the scene), they will signal to other security guards for back-up. At that time, there will be a congregation of burglars and security guards at the point of entry while the alarm continues to sound. This is a close analogy of how your innate immune system functions. Once physical barriers such as the skin are breached, cellular contents, bacterial toxins, cytokines, and other molecules can all act as alarm signals. The complement system is activated destroying some bacteria but also signaling and activating immune cells to the site (acting as the security guards on-site who call for more back-up). Patrolling macrophages and other phagocytes congregate at the area to clear damaged or infected cells while at the same time releasing cytokines themselves that signal to other immune cells that help is needed. The innate immune system in action can be observed as the redness and swelling (hallmarks of inflammation) that occur when someone has an allergic reaction to a substance. These symptoms are a direct result of the accumulation of white blood cells (forming pus) at the area, increased blood flow and permeability of blood vessel walls, and the release of histamine by activated basophils and mast cells that allows additional leukocytes and fluid entry at the infected site (swelling/redness). Leukocytes continue to the site while some cytokines activate the acute phase response if the infection is major. If it has been successfully suppressed, macrophages and some white blood cells remain at the site to clean and repair damage, but eventually the immune response returns to normal. All of this happens whether you have been exposed to the pathogen or not; but as you can imagine, a depressed ability of the innate system to respond would result in greater time for infection to take hold and spread.
Recall Jake from the case presentation at the beginning of this chapter (see Case Study). For some reason, he seemed to be getting small colds more often than his roommate Jerry. One of the reasons why individuals can catch a cold or flu several times a year is that there are several hundred strains of the cold or flu viruses and mutations during reproduction generate new ones each year. Antibodies generated against specific cold viruses will not work as effectively on new viruses. As a consequence, the ability to fight off the common cold is largely dependent on innate immune system. We can then suspect that, for some reason, Jake’s innate immune system is suppressed allowing these viruses to infect and multiply in his body, thereby causing more pronounced symptoms.
On the other hand, an enhanced innate immune system would result in more speedy suppression of infection and faster healing. Given that Jerry and Jake are roommates and are therefore likely exposed to similar pathogens, again we can suspect that there is something about Jerry’s innate immune system that is more active than Jake’s. Interestingly, with both acute exercise (i.e. a single bout) and chronic (long term) exercise training, the innate immune system is predominantly affected.
Recall, however, that dendritic cells can migrate to the site of infection by chemotaxis, phagocytose foreign material as part of the innate response, and then re-enter the circulation to finally “present” the digested antigenic material to immune cells in the lymph tissue. This, other APCs, and a more global spread of pathogens can activate B and T lymphocytes that mount more long term, sustained, and in some cases, lifelong defenses.
Acquired Immunity: Adaptation to Specific Threats
While all of the components of the acquired immune system are present in the body at all times, they are not functionally capable of successfully combating a pathogen until they “learn” about that threat. This learning and memory are important and distinguish the acquired from the innate system. Immune reactivity through the acquired immune system occurs in 3 stages:
- Recognition
- Amplification
- Memory
Recognition occurs when IgM antibodies on select circulating B cells (called clones) bind with free pathogen or when both B and T cells are presented with antigen from APCs.
In the case of B cells, antigen binding leads to their transformation into antibody secreting plasma cells which then divide and “expand” the clone of cells so that more antibodies against the specific pathogen can be made. This amplifies the response to the pathogen. Some B cells become memory cells that remain dormant but expand that specific clone of B cells for future use and the system is then deemed to have memorized the response to that antigen. In a subsequent infection, the B cell antibody response will be swifter, eliminating that threat sooner. The process of B cell differentiation and expansion takes several days, but the memory cells can last in the body for a lifetime.
While B cells primarily respond to free pathogen, T cells respond to infected host cells. Activation of both B and T cells normally occurs at the same time. As such, several days typically pass before the cell-mediated immunity of T cells can come into effect. Nonetheless, once infected cells are recognized by specific T cell clones, immune stimulating molecules (typically cytokines) activate these cells to expand and differentiate into TC cells and TH cells. TC cells disable and destroy infected cells as described earlier, while TH cells orchestrate a coordinated action of all parts of the immune system in the attack of the pathogen. These actions include stimulation of B cells, increased activity of TC cells, chemokine secretion to influence phagocyte migration and stationing at the site of infection, and stimulation of eosinophils in allergic responses. In this regard, TH cells are like the quarterbacks of the immune system and their proper functioning is vital to immune health. Some activated T cells also become memory T cells that are stationed around the body to elicit faster responses upon secondary exposure to that specific pathogen.
The ability to learn and remember foreign invaders is a powerful characteristic of the acquired immune system that allows us to mount defenses in a world where threats may be ever changing. As we shall see in the next section, exercise can have profound effects on the immune system. It is for this reason that the scarcity of research on exercise and acquired immune responses is surprising. Nonetheless, let’s now take a look at specific effects of exercise on immunity.
—
Gender Box: Females Have More Active Immune Systems
Interestingly, females of reproductive age tend to have more active immune systems including greater TH2 cytokines, more T lymphocytes, and a greater percentage of active neutrophils and macrophages. This may account for lower mortality rates after acute infection related inflammatory events, but higher rates of autoimmune diseases such as Lupus, Multiple Sclerosis and Rheumatoid Arthritis (Pennell et al. 2012). While estrogen has been linked to this sex dimorphism, other factors likely act in concert with it. Nonetheless, given the higher activity of the immune system in females, it is to prudent to ask what will exercise do to this response. While the research in this area is limited, it appears that at least some portions of the immune response are modulated by exercise. In particular, pubertal and post-pubertal women show increased NK cells immediately following moderate intensity exercise (Timmons et al. 2006). However, after an acute bout of moderate exercise (70% V ̇O2max) pre-pubertal girls exhibit a blunted leukocytosis.
—
Exercise Affects the Immune System
Strenuous exercise in the animal world typically involves life or death circumstances. The lion must eat and the buffalo must not be eaten, but for the lion and buffalo the possibility of torn skin, tissue damage, broken bones, and consequent susceptibility to disease or infection are dangers the body must prepare for. When the physiological effects of exercise are examined from this point of view, it is easy to see their importance. While it is no longer a requirement that we exercise vigorously under the auspices of mortal danger, the potential for injury is still inherent and the physiological responses that occur in modern day humans are the same nonetheless. In the following sections we will describe how exercise modifies the immune system components discussed previously. What may be most interesting is that these effects can at times be immune stimulating and at others immune inhibiting. Moreover, because exercise carries inherent risks of injury and close physical contact with others, recognition of infection probability is important. Nonetheless, the wealth of data on this subject would tend to support observation that light to moderate intensity exercise training improves immune function, especially that of the innate immune response. It appears that moderate exercise (walking 35-45 min per day in elderly men and women (Nieman 2008) or 60-80 min per week of moderate exercise in middle-aged adults (Matthews et al. 2002)) can significantly reduce the incidence of cold and cold symptoms, compared to sedentary people.
Contrast this with one of the most classic examples of the effects of over-training on the immune system and overall health of the body: the case of former American and World marathon record holder, multiple-time New York and Boston marathon winner and current USATF coach, Alberto Salazar. In the early 1980’s, Alberto Salazar was one of the most prolific marathoners in the world. After several years of intense training and a particularly grueling victory in the 1980 “Duel in the Sun” Boston marathon where Salazar required medical attention after the race, Alberto began training for the 1984 Olympic marathon. Salazar’s training regimen leading up to the event was reported to be several hundred miles of intense running every week. However, within this time Salazar also reported that he developed 12 colds in the 12 months of over-training. In fact, Salazar may not have known by what mechanisms this was occurring but he was quoted as saying “my immune system was totally shot as a result of my training”. This is a sentiment anecdotally shared by many high-performance athletes and has been found in the relevant research both in Olympic athletes as well as after a single prolonged and intense bout of exercise.

How can exercise at these two intensities differ so much in its effect on the immune system? As proposed by Dr. DC Nieman and others, the immune-modulating effects of exercise exist on a J-shaped curve with exercise intensity (Figure 15-8) such that moderate intensity exercise is more beneficial than light or no activity against infection, but intense exercise can actually be detrimental. The following sections highlight the components of the immune system that are modified by exercise in this regard.
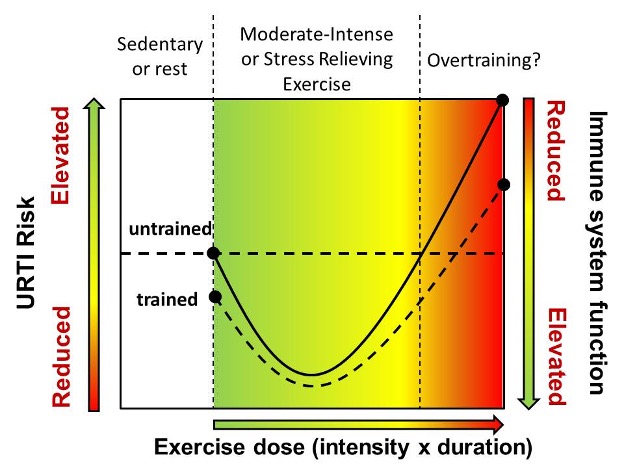
Exercise and Primary Barrier Support (Clotting)
Think about all the times you have ever scraped your knee or received a blow that broke the skin. Likely you were involved in some form of physical activity (playing tag in the schoolyard, a fall while skating or at the extreme end, a grueling boxing match, for example). Abrasions, blisters, and cuts/lacerations combined make up the largest percentage of injuries for individuals involved in running activities (Gosling et al. 2010). This is an inherent risk in physical activity, and while these injuries are typically considered minor, they do break the skin which we will recall is the primary barrier in the defense against foreign pathogens. In response to exercise of various intensities, there is an increase in the overall coagulation and thrombin generation potential of the blood (Menzel and Hilberg 2011) which is sustained for some period after exercise (Ikarugi et al. 2003). These results are primarily due to the sympathoadrenal catecholamines, epinephrine, and norepinephrine (Ikarugi et al. 1999) which are key mediators of the body’s fight-or-flight response during exercise. Further, catecholamine-stimulated platelet activation is augmented by a blood flow mediated increase in blood vessel shear stress that occurs with elevated cardiac output during exercise (Goto et al. 1996). This shear stress acts synergistically with the catecholamines to increas e platelet activation and increase clotting potential after exercise (Goto et al. 1996). Further, maximal intensity, short duration (90 sec) exercise may increase platelet activation (i.e. priming for response to some stimulus) (Hilberg et al. 2003), while moderate and strenuous exercise over an hour appears to increase the release of platelet and neutrophil derived microparticles (i.e. fragmented portions of the plasma membrane that can activate and recruit other leukocytes) in addition to platelet activation (Hilberg et al. 2002; Chaar et al. 2011). Interestingly, trained individuals show as much or a greater response of the microparticle increase (Sossdorf et al. 2011), but it appears that exercise training reduces the exercise induced shear stress related increase in procoagulation potential (Wang et al. 2005). The latter indicates the importance of exercise training in contrast to the effects of an acute exercise bout. Given that the intensity of exercise is important to the magnitude of procoagulation change (Hilberg et al. 2008), training may act to reduce the relative intensity of any given exercise bout and consequently reduce the catecholamine and shear induced increase in clotting potential.
Interestingly, the conjugation of platelets and leukocytes has also been observed after various exercise intensities (Hilberg et al. 2002, 2003). The meaning of this is unclear at this time, but this is an intriguing find, given that platelet-leukocyte conjugates are primarily observed at times of cardiovascular stress such as after ischaemic stroke (Marquardt et al. 2009) and acute coronary syndromes such as myocardial infarction (heart attacks) where they may increase vascular obstruction at plaque sites (Sarma et al. 2002).
As noted in the opening paragraph to this section, an increase in the clotting potential of humans undertaking physical exercise seems intuitive when viewed from the perspective of the fight-or-flight response. While exercise today is not typically performed under the auspices of life or death, the physiological responses are conserved from the times when it was. Scratches, gashes, and bites were to be expected, and these wounds could potentially result in significant blood loss and would also expose the body to potentially dangerous microbes. An enhanced ability to seal these wounds is a benefit. However, this increased clotting ability has also been theorized to be a cause of cardiovascular complications after exercise (https://www.ncbi.nlm.nih.gov/pubmed/12959620 Nonetheless, it is not uncommon to observe significant increases in the fibrinolytic potential of humans after exercise, and this result has been theorized to counter the potentially dangerous effects of a hyper-reactive clotting system (Weiss et al. 1998). Further, after multi week training programs of moderate intensity, fibrinolytic potential is improved in both young and old men and women but men tend to show a more pronounced response (Kulaputana et al. 2005). The reasons for this are unclear but may be due to differences in body and body composition changes as a result of exercise.
Exercise and Upper Respiratory Tract Infections
You may be asking yourself, “but I always thought exercise was good for you. The harder the better, right? No pain, no gain.” While a progressive increase in training intensity is a hallmark of inducing further physiological adaptations, the increase in training intensity does have it limits. With respect to exercise and immune function, the consequences of immunosuppression observed with prolonged high intensity exercise are notable. One of the most common medical conditions affecting Olympic athletes is succumbing to common upper respiratory tract infections (URTI). Olympic athletes and in particular those athletes who compete in the longest duration type events (e.g. marathon or triathlon) show a significantly elevated risk of URTI above sedentary individuals (https://www.ncbi.nlm.nih.gov/pubmed/26568028 ).
Exercise-Induced Asthma and Exercise-Induced Bronchoconstriction
Asthma is defined as a chronic inflammatory condition of the air passageways in the lungs. The lining of the airways may become inflamed, produce more mucous, or narrow due to tightening of the muscles surrounding the airways. The difficulty manifested by these physiological occurrences is obvious: a person experiencing asthma-like symptoms will find it difficult to move air into and out of the lungs. In individuals with asthma, triggers may either induce inflammation or tightening of the surrounding muscles around already inflamed tissue. While pollens, dust mites, mould, viral infections, and other air pollutants may be triggers for inflammation, exercise happens to be a major trigger of bronchiole muscle tightening (bronchospasms). In fact, while the term “exercise -induced asthma” (EIA) is still used today, referring to this event as exercise -induced bronchospasms (EIB) is more correct and inclusive of all individuals experiencing symptoms (Gotshall 2002). As a consequence of EIB, some individuals may experience extreme difficulty breathing during, after, and as a result of exercise, a time when unrestricted air transport is even more vital. Symptoms of EIB usually become greater with increased exercise intensity and duration, but it is important to note that this may not be the same as the increased ventilation (heavy breathing) or dyspnea (shortness of breath) you may have experienced during intense activity that returned to normal a short time after exercise was completed. Exercise induced asthma is a much more severe and longer lasting respiratory distress and is actually a leading cause of asthmatic patients first presentation to a medical professional (Parsons 2010). Nonetheless, while exercise-induced asthma is common in individuals who have been clinically diagnosed with asthma (40-90% of asthmatics (McFadden and Gilbert 1994; Parsons et al. 2011), this is not a requirement. It has been reported that approximately 26% of non-asthmatic people report symptoms (shortness of breath, wheezing, coughing, difficulty inhaling, or chest tightness) that are consistent with EIB after exercise (Parsons et al. 2011). These incident rates should not be taken lightly. In one of the only studies to examine exercise and asthma related deaths, it was determined that over a seven-year period (1993-2000) in the United States, 61 deaths of individuals competing in athletic activity could directly be attributed to EIA or EIB while another 202 may have been associated with EIB symptoms (Becker et al. 2004).
Exercise Increases Concentration of Circulating White Blood Cells
As early as 1893, the increased concentration of white blood cells (leukocytosis) was observed to occur in humans undergoing strenuous work. A slew of articles in the next few decades examined this phenomenon and were summarized nicely, along with additions, in a paper by Doctors. H.T. Edwards and W.B. Wood out of the Harvard Fatigue Laboratory (Edwards and Wood 1932). Summarizing the effects of vigorous physical activity in soldiers, football players, marathoners, 400 m sprinters, and hockey players on leukocytosis, it was shown that this effect occurred in both trained and untrained individuals and was dependent on exercise duration on exercise duration and intensity. Further, approximately one hour after exercise there was a marked decline in plasma lymphocyte expression (below normal) that appeared to be due to storage of these cells rather than loss. These results have been repeated since this time.
An important consideration here is that vigorous exercise involves significant water loss and consequently plasma volume loss which would tend to concentrate the other constituents of blood independent of new secretion. This potentially confounding factor was addressed early on but ruled unlikely given the lack of a correlation between mass and water loss and the extent of leukocytosis. Currently, it is clear that this exercise induced leukocytosis is the result of newly secreted leukocytes, primarily as a result of adrenal catecholamines. Not only are the immune cells increased in concentration in the plasma after exercise, but they also appear to be primed such that they will respond to the same stressor with greater activity (Degerstrøm and Østerud 2006).
In this regard, it is important that hormonal responses occurring during exercise and their effects on immunity are presented here.
Exercise Related Hormones and Cytokines Affect Immune Cells
Neuroendocrine Chemical Messengers Mediate Immune System Changes During Exercise
The sympathoadrenal, hypothalamus-pituitary adrenal and growth hormone axes, and some natural opiates are all elevated in an intensity and duration dependent manner during exercise (Luger et al. 1987). Together these hormones include the catecholamines (norepinephrine and epinephrine), cortisol, growth hormone, prolactin, and β-endorphin. The increase in these hormones, especially the catecholamines, prior to any observed leukocytosis or cytokine release is indicative of their roles in immune function. Each hormone has different physiological roles during exercise including the elevation of cardiac output, regulation of blood distribution, maintenance of glucose homeostasis, and regulation of metabolic substrate availability and use. These hormones cause physiologic changes that resist the multitude of stresses that may occur during exercise. Hormones exert their effects by working on receptors in target cells. Consequently, if a particular cell possesses a receptor for some hormone, it can respond to that particular hormone.
The Catecholamines
The sympathetic nervous system (think fight-or-flight response) innervates most organs and tissues of the immune system (Mignini et al. 2003). In fact, lymphoid tissues and all leukocytes possess adrenergic receptors that respond to catecholamines released from the sympathetic nervous system or adrenal catecholamines in the circulation (Elenkov et al. 2000). Catecholamine stimulation causes movement of white blood cells out of the circulation and aids in activation of these cells. The difference in the densities of the different types of adrenergic receptors on immune cells dictates their responsiveness to catecholamine stimulation. For example, NK cells have a higher proportion of β2-adrenergic receptors than T-cells, and consequently, NK cells show greater changes (i.e. increased movement into the circulation and enhanced activity) in response to catecholamine stimulation (Elenkov et al. 2000). During moderate activity, sympathetic norepinephrine primarily stimulates leukocytosis, but epinephrine becomes the predominant mechanism during intense exercise. Nonetheless, acute exposure to the catecholamines stimulates leukocytosis and enhanced immune function (Krüger et al. 2008).
Cortisol
Cortisol, the primary glucocorticoid, is a powerful glucoregulatory steroid hormone. It is also an anti-inflammatory steroid. Cortisol invokes its anti-inflammatory actions by:
- increasing the amount of anti-inflammatory molecules and
- decreasing the transcription of pro-inflammatory eicosanoids, cytokines, and chemokines
Cortisol also inhibits immune function by:
- causing a redistribution of circulating cells to the bone marrow;
- •reducing lymphocyte movement from peripheral lymph tissues to the circulation;
- inhibiting NK cell function; and
- inducing B and T cell apoptosis (supraphysiological doses)
Because of these roles, it is not surprising that many athletes receive glucocorticoid injections in the form of hydrocortisone injections or topical creams to relieve joint inflammation. In fact, exogenous glucocorticoids are a type of the most commonly detected substances in high level athletes by the World Anti-Doping Agency. Cortisol is a steroid and many of its actions work on the genome, making them slower responding than those of the catecholamines. Further, cortisol is primarily elevated during prolonged exercise or when blood glucose levels drop significantly. For these reasons, the actions of cortisol on the immune system are likely more predominant during the recovery period after exercise. Indeed, its immunosuppressive roles would fit with the decrease in circulating lymphocytes observed after an acute bout of exercise.
Growth Hormone
One of the most potent inducers of an increase in plasma growth hormone is physical exercise. Like the other hormones, it is elevated in an exercise dose (intensity x duration) manner where it provides additional metabolic support by increasing peripheral availability/use and helps in repair. Unlike the adrenal catecholamin es, growth hormone typically peaks near the end or even after strenuous exercise. Thus, its roles are more delayed. Nonetheless, growth hormone also contributes to leukocytosis through growth hormone receptors on most cells of the immune system. To this effect, growth hormone stimulates or enhances:
- neutrophil differentiation
- •lymphocyte proliferation, activation, and migration
- macrophage activation
While growth hormone is primarily secreted by the anterior pituitary gland, it is now apparent that most immune cells can secrete it as well (Clark 1997). As such, growth hormone is thought to act in local immune signalling either directly or through the mediator of growth hormone action, insulin-like growth factor (IGF-1). Further, several cytokines act on the hypothalamus and pituitary gland to modify growth hormone production.
Endogenous Opiates
The more common opiates you might have heard of are exogenous analogs such as morphine or illegal drugs such as heroin. When you think about what these drugs are used and abused for, it should not be surprising that the opiates are analgesic and may cause feelings of euphoria. The common endogenous opiates are the endorphins and enkephalins of which β-endorphin is most commonly studied during exercise. The exact role of β-endorphin in exercise is not entirely clear, but it is associated with an extreme euphoric sensation some runners experience during prolonged or intense exercise known as the runner’s high. The time-course of increase in β-endorphins tends to match that of cortisol because it is synthesized from a large pro-hormone that also contains the tropic hormone for cortisol release. Like many of the hormones, an immunomodulating role for β-endorphin has been observed in vitro, but this is not as clear inside the body. Nonetheless, the endogenous opiates have both immunostimulating and immunosuppressive effects on lymphocyte activity. For example, high levels of β-endorphin are directly correlated with increased NK cell activity while high enkephalin appears to suppress it at rest (Mozzanica et al. 1991). Further, the lymphocyte changes in response to increases in the endogenous opiates is small (Carr and Serou 1995), but they cannot be fully discounted as playing a role in the exercise-induced changes in the immune system.
Glutamine Availability
Glutamine, an abundant non-essential amino acid, is a key substrate used by lymphocytes and monocytes for energy and cell proliferation. Lymphocytes utilize glutamine several folds greater than glucose to meet their energy demands. In fact, glutamine supplementation has an extensive history in boosting the immune system in clinical populations (Ziegler 1992; Estívariz et al. 2008). Skeletal muscle is the largest source of plasma glutamine (Newsholme and Parry-Billings 1990). Further, strenuous exercise has been shown to reduce muscle and plasma glutamine levels (Kargotich et al. 2005) in a timeframe similar to reduced immune system function. Not surprisingly, it has been theorized that the maintenance of plasma glutamine would prevent immunodepression with exercise. Indeed, some reports have shown improved immune function in athletes supplementing with either glutamine (Castell and Newsholme 1997) or branch chain amino acids that prevented a fall in glutamine during strenuous exercise (Bassit et al. 2000). However, it has also been shown that maintenance of glutamine levels is not sufficient to maintain immune function in all cases (Mackinnon and Hooper 1996; Delgado et al. 2006), though this does not preclude glutamine availability as one of many factors that affect immune function.
Delayed Onset Muscle Soreness After Exercise: Role of the Immune System
Exercise, especially intense or eccentric type activity, may induce significant muscle fibre damage including disruption to the myofibre sarcolemma, proteins, cytoskeleton, and structural components between the myofibres (Fridén and Lieber 2001). Attracted by the products of protein degradation and inflammatory cytokines released by damaged cells, neutrophils followed by macrophages invade the damaged area to clear cellular debris and help in the repair and regeneration of damaged skeletal muscle (Tsivitse et al. 2003). Indeed, there is a concomitant increase in plasma leukocytes (neutrophils, macrophages, and NK cells) and skeletal muscle neutrophils, macrophages, and activated satellite cells in the days following an acute damaging exercise bout and multiple biopsies alone (Malm et al. 2000). Either muscle cells or infiltrating leukocytes may then release inflammatory cytokines (predominantly IL-1, IL-6, and/or TNFα) that act as chemotaxic signals for other immune cells. Indeed, exercise associated with significant myofibre damage invokes a low grade inflammatory response that includes increased circulating cytokines and leukocytes for up to several days later although these immune changes do not always correspond with the common feelings of delayed onset muscle soreness (Kuipers 1994). The time-course of change after muscle damage is shown in Figure 15-9.
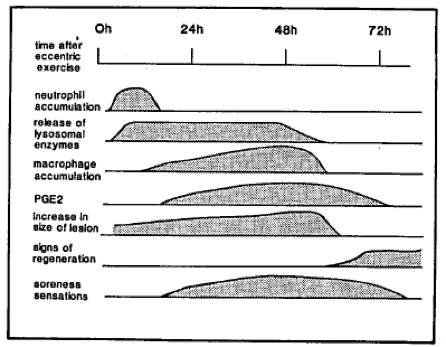
Exercise and Endotoxemia
As noted above, we normally live in a symbiotic relationship with a variety of beneficial bacteria. The greatest concentration of these bacteria is found in the large intestines. The reason these bacteria are not normally pathogenic is because we maintain adequate physical (mucosal and cellular) and immunological (intestinal lymph nodes) barriers to their entrance into the body. These barriers are maintained by a healthy physiology. Under stress (for example in burn or shock victims) where there is a reduction in splanchnic blood flow, there is the potential for the gastrointestinal mucosal barrier to succumb to ischaemic injury. Lack of oxygen and nutrient rich blood may lead to cell death. Once this barrier is compromised, normal flora may be able to penetrate into the body’s circulation invoking systemic inflammatory responses directed against the bacteria and/or their toxins (endotoxemia). During exercise, blood flow is redirected away from non-essential organs such as the intestines in order to satisfy the nutrient and gas needs of working muscle as well as essential organs like the brain and lungs. It has been theorized that this exercise induced blood redirection may be a source of intestinal ischaemic injury and subsequently endotoxemia (Marshall 1998; Pedersen et al. 1998). These theories are supported by the gastrointestinal distress symptoms commonly observed in athletes participating in long duration high intensity exercise. For example, symptoms such as dizziness, stomach cramps, nausea, vomiting, and diarrhea (with and without blood in the stool) are all highly reported in marathon runners (Riddoch and Trinick 1988), ultramarathon runners (Rehrer et al. 1992), triathletes (Peters et al. 1999), and Ironman triathletes (Pfeiffer et al. 2012). Further, endurance athletes show increased plasma concentrations of endotoxins and anti-endotoxin IgG immediately after exercise and these observations are significantly correlated with magnitude of gastrointestinal distress symptoms (Brock-Utne et al. 1988). However, even though splanchnic blood flow has been shown to be reduced during exercise, several reports show a disconnect between this reduced blood flow and symptomology (Wright et al. 2011). Consequently, there are likely other factors that cause, independently or in conjunction with reduced blood flow, the gastrointestinal symptoms observed in some athletes.
Exercise and the Acute Phase Response
Training reduces plasma C reactive protein (CRP) levels in healthy adults (Lakka et al. 2005). In fact, one of the strongest correlates with reduced CRP is maximal oxygen uptake suggesting that one of the adaptations associated with fitness is a reduction in whole body stress. Because this protein is associated with many chronic diseases including Type 2 diabetes and heart disease (Lakka et al. 2005), reductions in CRP with training could account for portions of the reduction in Type 2 diabetes and CVD risk as a result of exercise.
Interestingly, Dr. BK Pedersen has shown over a series of studies that the clinical syndrome known as the systemic inflammatory response syndrome, essentially a global, highly negative inflammatory state, is reproduced very closely by vigorous exercise. What should be questioned here is, why do stresses such as trauma, burns, and serious infection result in life threatening immune response, while exercise with a similar cytokine and immune profile typically does not?
The answer to the question above is that while the cytokines and activated immune cells are similar during exercise and other pathological stresses, the magnitude and duration of the immune response is much less as a result of exercise. These changes are shown in Figure 15-10. For example, the exercise-induced increase in plasma IL-6 is not even elevated to a tenth of the level associated with mortality during sepsis (~40pgml-1 after strenuous exercise (Ostrowski et al. 1998a) versus ~280-950 pgml-1 during sepsis causing 50% mortality (Casey et al. 1993). Further, in contrast to serious infection, the cytokine response to strenuous exercise (e.g. marathon running at ~75% of V ̇O2max) favours anti-inflammatory molecules. Plasma levels of the inflammatory cytokines TNFα and IL-1 are minimally changed after prolonged strenuous exercise (Ostrowski et al. 1998a) and the levels do not approach those observed in sepsis (Casey et al. 1993). Moreover, IL-10, IL-1 receptor antagonist (IL-1ra) and soluble TNF- receptor are elevated subsequent to the increase in IL-6 (Ostrowski et al. 1998a, 1999). The cytokine IL-10 is a potent anti-inflammatory cytokine while IL-1ra and sTNFα-R decrease the signalling capabilities of the pro-inflammatory cytokines, IL-1, and TNFα by limiting their ability to interact with receptors on other immune and non-immune target cells.
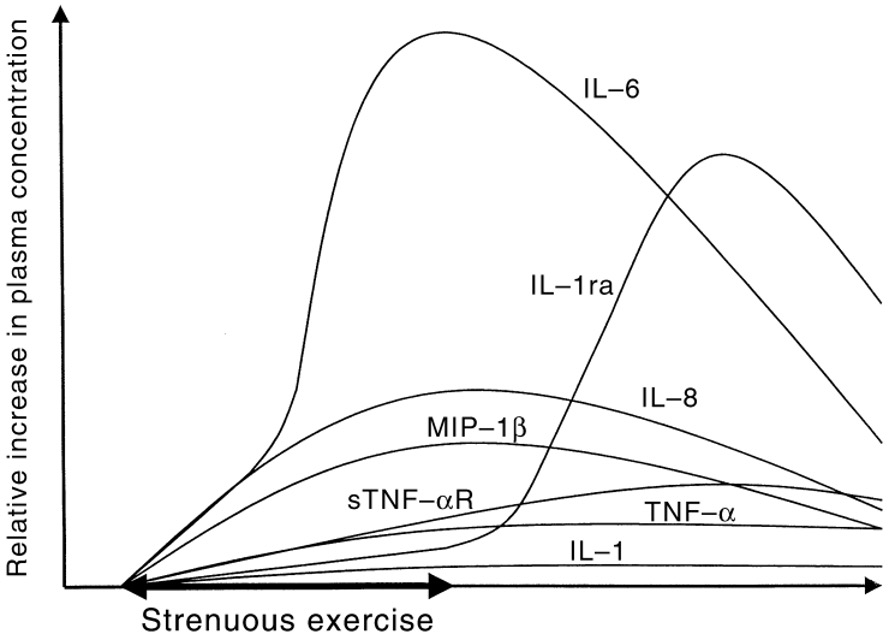
Although the purpose of an increase in IL-6 is not completely known, its apparent source during exercise is contracting skeletal muscle (Steensberg et al. 2002) rather than circulating immune cells. This increase tends to both correlate with (Bruunsgaard et al. 1997) and occur independent of (Ostrowski et al. 1998b; Toft et al. 2002) markers of significant muscle damage. However, in addition to its role in inflammation, exercise-induced plasma IL-6 functions like a muscle derived hormone to increase hepatic glucose output and assist in muscle adaptation (Pedersen et al. 2003). With respect to the former, this is partly due to the IL-6 stimulated increase in the glucoregulatory and anti-inflammatory hormone, cortisol (Steensberg et al. 2003). As such, glucose feeding during intense exercise can reduce the IL-6 increase (Febbraio et al. 2003).
Further evidence for a differential effect between exercise and severe infection is that exercise does not appear to affect the complement system (Córdova et al. 2010). Consequently, the cascading physiological responses of the immune system to infection (e.g. fever associated with the acute phase response) do not tend to occur with exercise.
Summary
It is quite apparent that exercise has profound effects on the immune system. These effects are dependent on exercise intensity, proper training regimens (i.e. the avoidance of over-training), and likely good nutrition. Exercise is a physiological stress and the repeated disturbance to homeostasis of several physiological variables as a result of exercise training may lead to adaptations that fortify the body against those stresses. When exercise training is accomplished from the standpoint of health promotion, these adaptations are overwhelmingly positive. This includes stimulation and priming of the immune system observed in low-to-moderate intensity training. However, competitive athletes often walk a fine line between training that will induce positive adaptations and over-training. With respect to immune system dysfunction, it is the high training intensities with little rest and elevated stress hormone levels (i.e. cortisol and epinephrine) that appears to be detrimental to immune function. Immune suppression can be observed after as little as a single bout of intense and/or prolonged exercise and last for days to weeks. While not isolated to the competitive athlete, suppression of the immune system is exacerbated by psychological and other physiological stresses (e.g. Olympic athletes exhibit poorer quality of sleep than age and sex matched controls (Leeder et al. 2012) ) that are likely to be higher in elite athletes. In particular, this immune suppression is associated with the increased risk of upper respiratory tract infection in elite athletes, one of the most common medical conditions in Olympic level athletes. Consequently, it is important for both the recreational and elite athlete to be aware of the potential immune system suppression of intense prolonged exercise, so that they may take the appropriate measures to try to limit these negative effects.
Review Questions
References
Asea, A., Rehli, M., Kabingu, E., Boch, J.A., Baré, O., Auron, P.E., Stevenson, M.A., and Calderwood, S.K. 2002. Novel Signal Transduction Pathway Utilized by Extracellular HSP70. Journal of Biological Chemistry 277(17): 15028–15034. Elsevier BV. doi:10.1074/jbc.m200497200.
Auger, P., Marquis, G., Joly, J., and Attye, A. 1993. Epidemiology of tinea pedis in marathon runners: prevalence of occult athlete’s foot. Mycoses 36(1–2): 35–41. doi:10.1111/j.1439-0507.1993.tb00685.x.
Bassit, R.A., Sawada, L.A., Bacurau, R.F., Navarro, F., and Costa Rosa, L.F. 2000. The effect of BCAA supplementation upon the immune response of triathletes. Med Sci Sports Exerc 32(7): 1214–9. doi:10.1097/00005768-200007000-00005.
Becker, J.M., Rogers, J., Rossini, G., Mirchandani, H., and D’Alonzo Jr, G.E. 2004. Asthma deaths during sports☆Report of a 7-year experience. Journal of Allergy and Clinical Immunology 113(2): 264–267. Elsevier BV. doi:10.1016/j.jaci.2003.10.052.
Berg, R. 1996. The indigenous gastrointestinal microflora. Trends Microbiol 4(11): 430–435. Elsevier BV. doi:10.1016/0966-842x(96)10057-3.
Bouvet, J.P., and Fischetti, V.A. 1999. Diversity of antibody-mediated immunity at the mucosal barrier. Infect Immun 67(6): 2687–2691. United States. doi:10.1128/IAI.67.6.2687-2691.1999.
Brenner, I.K., Shek, P.N., and Shephard, R.J. 1994. Infection in athletes. Sports Med 17(2): 86–107. New Zealand. doi:10.2165/00007256-199417020-00002.
Brock-Utne, J.G., Gaffin, S.L., Wells, M.T., Gathiram, P., Sohar, E., James, M.F., Morrell, D.F., and Norman, R.J. 1988. Endotoxaemia in exhausted runners after a long-distance race. South African Medical Journal 73(9): 533–536.
Bruunsgaard, H., Galbo, H., Halkjaer-Kristensen, J., Johansen, T.L., MacLean, D.A., and Pedersen, B.K. 1997. Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. J Physiol 499 ( Pt 3)(Pt 3): 833–841. England. doi:10.1113/jphysiol.1997.sp021972.
Butterfield, T. a, Best, T.M., and Merrick, M. a. 2006. The dual roles of neutrophils and macrophages in inflammation: A critical balance between tissue damage and repair. J Athl Train 41(4): 457–465.
Calderwood, S.K., Gong, J., Theriault, J.R., Mambula, S.S., and Gray, P.J. 2008. Cell stress proteins: novel immunotherapeutics. Novartis Found Symp 291: 115–31; discussion 131-40. doi:10.1002/9780470754030.ch9.
Carr, D.J.J., and Serou, M. 1995. Exogenous and endogenous opioids as biological response modifiers. Immunopharmacology 31(1): 59–71. Elsevier BV. doi:10.1016/0162-3109(95)00033-6.
Casey, L.C., Balk, R.A., and Bone, R.C. 1993. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med 119(8): 771–8. doi:10.7326/0003-4819-119-8-199310150-00001.
Castell, L.M., and Newsholme, E.A. 1997. The effects of oral glutamine supplementation on athletes after prolonged, exhaustive exercise. Nutrition 13(7–8): 738–742. Elsevier BV. doi:10.1016/s0899-9007(97)83036-5.
Chaar, V., Romana, M., Tripette, J., Broquere, C., Huisse, M.-G., Hue, O., Hardy-Dessources, M.-D., and Connes, P. 2011. Effect of strenuous physical exercise on circulating cell-derived microparticles. Clin Hemorheol Microcirc 47(1): 15–25. IOS Press. doi:10.3233/ch-2010-1361.
Clark, R. 1997. The Somatogenic Hormones and Insulin-Like Growth Factor-1: Stimulators of Lymphopoiesis and Immune Function. Endocr Rev 18(2): 157–179. The Endocrine Society. doi:10.1210/edrv.18.2.0296.
Collaboration, A.T.C. 2010. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996-2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis 50(10): 1387–1396. United States. doi:10.1086/652283.
Córdova, A., Sureda, A., Tur, J.A., and Pons, A. 2010. Immune response to exercise in elite sportsmen during the competitive season. J Physiol Biochem 66(1): 1–6. Springer Science and Business Media LLC. doi:10.1007/s13105-010-0001-2.
Crawford, F. 2009. Athlete’s foot. BMJ Clin Evid 2009.
Degerstrøm, J., and Østerud, B. 2006. Increased inflammatory response of blood cells to repeated bout of endurance exercise. Med Sci Sports Exerc 38(7): 1297–1303. doi:10.1249/01.mss.0000227315.93351.8d.
Delgado, L., Fonseca, J., Korpela, R., Kekkonen, R.A., Moreira, A., and Haahtela, T. 2006. Nutritional modulation of exercise-induced immunodepression in athletes: a systematic review and meta-analysis. Eur J Clin Nutr 61(4): 443–460. doi:10.1038/sj.ejcn.1602549.
Edwards, H.T., and Wood, W.B. 1932. A study of leukocytosis in exercise. Arbeitsphysiologie 6(1–2): 73–83. Springer Science and Business Media LLC. doi:10.1007/bf02009854.
Elenkov, I.J., Wilder, R.L., Chrousos, G.P., and Vizi, E.S. 2000. The sympathetic nerve – An integrative interface between two supersystems: The brain and the immune system.
Estívariz, C.F., Griffith, D.P., Luo, M., Szeszycki, E.E., Bazargan, N., Dave, N., Daignault, N.M., Bergman, G.F., McNally, T., Battey, C.H., Furr, C.E., Hao, L., Ramsay, J.G., Accardi, C.R., Cotsonis, G.A., Jones, D.P., Galloway, J.R., and Ziegler, T.R. 2008. Efficacy of parenteral nutrition supplemented with glutamine dipeptide to decrease hospital infections in critically ill surgical patients. JPEN J Parenter Enteral Nutr 32(4): 389–402. United States. doi:10.1177/0148607108317880.
Febbraio, M.A., Ott, P., Nielsen, H.B., Steensberg, A., Keller, C., Krustrup, P., Secher, N.H., and Pedersen, B.K. 2002. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol 544(3): 957–962. England. doi:10.1113/jphysiol.2002.025148.
Febbraio, M.A., Steensberg, A., Keller, C., Starkie, R.L., Nielsen, H.B., Krustrup, P., Ott, P., Secher, N.H., and Pedersen, B.K. 2003. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J Physiol 549(Pt 2): 607–612. England. doi:10.1113/jphysiol.2003.042374.
Fiala, K.A., Hoffmann, S.J., and Ritenour, D.M. 2003. A Survey of Team Physicians on the Participation Status of Hemophilic Athletes in National Collegiate Athletic Association Division I Athletics. J Athl Train 38(3): 245–251.
Field, L.A., and Adams, B.B. 2008. Tinea pedis in athletes. Int J Dermatol 47(5): 485–492. Wiley. doi:10.1111/j.1365-4632.2008.03443.x.
Fridén, J., and Lieber, R.L. 2001. Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol Scand 171(3): 321–326. Wiley. doi:10.1046/j.1365-201x.2001.00834.x.
Galli, S.J. 2000. Mast cells and basophils. Curr Opin Hematol 7(1): 32–39. Ovid Technologies (Wolters Kluwer Health). doi:10.1097/00062752-200001000-00007.
Garrett, W.S., Gordon, J.I., and Glimcher, L.H. 2010. Homeostasis and inflammation in the intestine. Cell 140(6): 859–870. United States. doi:10.1016/j.cell.2010.01.023.
Gleeson, M., and Pyne, D.B. 2000. Exercise effects on mucosal immunity. Immunol Cell Biol 78(5): 536–544. Wiley. doi:10.1111/j.1440-1711.2000.t01-8-.x.
Gordon, S., and Taylor, P.R. 2005. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5(12): 953–964. Springer Science and Business Media LLC. doi:10.1038/nri1733.
Gosling, C.M., Forbes, A.B., McGivern, J., and Gabbe, B.J. 2010. A Profile of Injuries in Athletes Seeking Treatment during a Triathlon Race Series. Am J Sports Med 38(5): 1007–1014. SAGE Publications. doi:10.1177/0363546509356979.
Goto, S., Handa, S., Takahashi, E., Abe, S., Handa, M., and Ikeda, Y. 1996. Synergistic effect of epinephrine and shearing on platelet activation. Thromb Res 84(5): 351–9. doi:10.1016/s0049-3848(96)00199-5.
Gotshall, R.W. 2002. Exercise-Induced Bronchoconstriction. Drugs 62(12): 1725–1739. Springer Science and Business Media LLC. doi:10.2165/00003495-200262120-00003.
Hallstrand, T.S., Moody, M.W., Wurfel, M.M., Schwartz, L.B., Henderson Jr, W.R., and Aitken, M.L. 2005. Inflammatory basis of exercise-induced bronchoconstriction. Am J Respir Crit Care Med 172(6): 679–686. United States. doi:10.1164/rccm.200412-1667OC.
Hilberg, T., Gläser, D., Schmidt, V., Lösche, W., Franke, G., Schneider, K., and Gabriel, H.H.W. 2003. Short-term exercise and platelet activity, sensitivity to agonist, and platelet-leukocyte conjugate formation. Platelets 14(2): 67–74. Informa UK Limited. doi:10.1080/0953710021000057541-1.
Hilberg, T., Menzel, K., Gläser, D., Zimmermann, S., and Gabriel, H.H.W. 2008. Exercise intensity: Platelet function and platelet–leukocyte conjugate formation in untrained subjects. Thromb Res 122(1): 77–84. Elsevier BV. doi:10.1016/j.thromres.2007.08.018.
Hilberg, T., Schmidt, V., Gläser, D., Schammne, D., Lösche, W., and Gabriel, H.H.W. 2002. Platelet activity, sensitivity to agonist, and platelet-leukocyte conjugate formation after long-term exercise. Platelets 13(5–6): 273–277. Informa UK Limited. doi:10.1080/0953770021000007249.
Horn, P., Kalz, A., Lim, C.L., Pyne, D., Saunders, P., Mackinnon, L., Peake, J., and Suzuki, K. 2007. Exercise-recruited NK cells display exercise-associated eHSP-70. Exerc Immunol Rev 13.
Ikarugi, H., Shibata, M., Shibata, S., Ishii, H., Taka, T., and Yamamoto, J. 2003. High Intensity Exercise Enhances Platelet Reactivity to Shear Stress and Coagulation during and after Exercise. Pathophysiol Haemost Thromb 33(3): 127–133. S. Karger AG. doi:10.1159/000077820.
Ikarugi, H., Taka, T., Nakajima, S., Noguchi, T., Watanabe, S., Sasaki, Y., Haga, S., Ueda, T., Seki, J., and Yamamoto, J. 1999. Norepinephrine, but not epinephrine, enhances platelet reactivity and coagulation after exercise in humans. J Appl Physiol 86(1): 133–138. American Physiological Society. doi:10.1152/jappl.1999.86.1.133.
Kargotich, S., Goodman, C., Dawson, B., Morton, A.R., Keast, D., and Joske, D.J.L. 2005. Plasma Glutamine Responses to High-Intensity Exercise Before and After Endurance Training. Research in Sports Medicine 13(4): 287–300. Informa UK Limited. doi:10.1080/15438620500359729.
Kita, H. 1996. The eosinophil: a cytokine-producing cell? J Allergy Clin Immunol 97(4): 889–92. doi:10.1016/s0091-6749(96)80061-3.
Klein, J. 1999. Self-nonself discrimination, histoincompatibility, and the concept of immunology. Immunogenetics 50(3–4): 116–123. Springer Science and Business Media LLC. doi:10.1007/s002510050587.
Krüger, K., Lechtermann, A., Fobker, M., Völker, K., and Mooren, F.C. 2008. Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav Immun 22(3): 324–338. Elsevier BV. doi:10.1016/j.bbi.2007.08.008.
Kuipers, H. 1994. Exercise-Induced Muscle Damage. Int J Sports Med 15(03): 132–135. Georg Thieme Verlag KG. doi:10.1055/s-2007-1021034.
Kulaputana, O., Macko, R.F., Ghiu, I., Phares, D.A., Goldberg, A.P., and Hagberg, J.M. 2005. Human gender differences in fibrinolytic responses to exercise training and their determinants. Exp Physiol 90(6): 881–887. Wiley. doi:10.1113/expphysiol.2005.030718.
Lakka, T.A., Lakka, H.-M., Rankinen, T., Leon, A.S., Rao, D.C., Skinner, J.S., Wilmore, J.H., and Bouchard, C. 2005. Effect of exercise training on plasma levels of C-reactive protein in healthy adults: the HERITAGE Family Study. Eur Heart J 26(19): 2018–2025. Oxford University Press (OUP). doi:10.1093/eurheartj/ehi394.
Lancaster, G.I., Møller, K., Nielsen, B., Secher, N.H., Febbraio, M.A., and Nybo, L. 2004. Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones 9(3): 276–280. Netherlands. doi:10.1379/csc-18r.1.
Leeder, J., Glaister, M., Pizzoferro, K., Dawson, J., and Pedlar, C. 2012. Sleep duration and quality in elite athletes measured using wristwatch actigraphy. J Sports Sci 30(6): 541–545. doi:10.1080/02640414.2012.660188.
Liles, W.C., and van Voorhis, W.C. 1995. Review: Nomenclature and Biologic Significance of Cytokines Involved in Inflammation and the Host Immune Response. Journal of Infectious Diseases 172(6): 1573–1580. Oxford University Press (OUP). doi:10.1093/infdis/172.6.1573.
Luger, A., Deuster, P.A., Kyle, S.B., Gallucci, W.T., Montgomery, L.C., Gold, P.W., Loriaux, D.L., and Chrousos, G.P. 1987. Acute Hypothalamic–Pituitary–Adrenal Responses to the Stress of Treadmill Exercise. New England Journal of Medicine 316(21): 1309–1315. Massachusetts Medical Society. doi:10.1056/nejm198705213162105.
MacKinnon, L.T., and Hoooper, S.U.E.L. 1996. Plasma glutamine and upper respiratory tract infection during intensified training in swimmers. Medicine & Science in Sports & Exercise 28(3): 285–290. Ovid Technologies (Wolters Kluwer Health). doi:10.1097/00005768-199603000-00003.
MacNeil, B., and Hoffman-Goetz, L. 1993. Chronic exercise enhances in vivo and in vitro cytotoxic mechanisms of natural immunity in mice. J Appl Physiol 74(1): 388–395. American Physiological Society. doi:10.1152/jappl.1993.74.1.388.
Malm, C., Nyberg, P., Engstrom, M., Sjodin, B., Lenkei, R., Ekblom, B., and Lundberg, I. 2000. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol 529 Pt 1(Pt 1): 243–262. England. doi:10.1111/j.1469-7793.2000.00243.x.
Marquardt, L., Anders, C., Buggle, F., Palm, F., Hellstern, P., and Grau, A.J. 2009. Leukocyte-platelet aggregates in acute and subacute ischemic stroke. Cerebrovascular Diseases 28(3): 276–282. doi:10.1159/000228710.
Marshall, J.C. 1998. The gut as a potential trigger of exercise-induced inflammatory responses. Can J Physiol Pharmacol 76(5): 479–484. Canadian Science Publishing. doi:10.1139/y98-049.
Matthews, C.E., Ockene, I.R.A.S., Freedson, P.S., Rosal, M.C., Merriam, P.A., And Hebert, J.R. 2002. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Medicine & Science in Sports & Exercise 34(8): 1242–1248. Ovid Technologies (Wolters Kluwer Health). doi:10.1097/00005768-200208000-00003.
McFadden, E.R., and Gilbert, I.A. 1994. Exercise-Induced Asthma. New England Journal of Medicine 330(19): 1362–1367. Massachusetts Medical Society. doi:10.1056/nejm199405123301907.
Menzel, K., and Hilberg, T. 2011. Blood coagulation and fibrinolysis in healthy, untrained subjects: Effects of different exercise intensities controlled by individual anaerobic threshold. Eur J Appl Physiol 111(2): 253–260. doi:10.1007/s00421-010-1640-2.
Mignini, F., Streccioni, V., and Amenta, F. 2003. Autonomic innervation of immune organs and neuroimmune modulation. Auton Autacoid Pharmacol 23(1): 1–25. Wiley. doi:10.1046/j.1474-8673.2003.00280.x.
Mozzanica, N., Finzi, A.F., Foppa, S., Vignati, G., and Villa, M.L. 1991. Association between circadian rhythms of endogenous hypothalamic opioid peptides and of natural killer cell activity. Int J Immunopharmacol 13(2–3): 317–321. Elsevier BV. doi:10.1016/0192-0561(91)90113-l.
Murshid, A., Gong, J., Stevenson, M.A., and Calderwood, S.K. 2011. Heat shock proteins and cancer vaccines: developments in the past decade and chaperoning in the decade to come. Expert Rev Vaccines 10(11): 1553–1568. England. doi:10.1586/erv.11.124.
Newsholme, E.A., and Parry-Billings, M. 1990. Properties of Glutamine Release From Muscle and Its Importance for the Immune System. Journal of Parenteral and Enteral Nutrition 14(4_suppl): 63S-67S. Wiley. doi:10.1177/014860719001400406.
Nieman, D., Nehlsen-Cannarella, S., Markoff, P., Balk-Lamberton, A., Yang, H., Chritton, D., Lee, J., and Arabatzis, K. 1990. The Effects of Moderate Exercise Training on Natural Killer Cells and Acute Upper Respiratory Tract Infections. Int J Sports Med 11(06): 467–473. Georg Thieme Verlag KG. doi:10.1055/s-2007-1024839.
Nieman, D.C. 2008. Physical activity benefits to the aging immune system. Agro Food Ind Hi Tech 19(5).
Ostrowski, K., Hermann, C., Bangash, A., Schjerling, P., Nielsen, J.N., and Pedersen, B.K. 1998a. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol 513 ( Pt 3)(Pt 3): 889–894. England. doi:10.1111/j.1469-7793.1998.889ba.x.
Ostrowski, K., Rohde, T., Asp, S., Schjerling, P., and Pedersen, B.K. 1999. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 515 ( Pt 1)(Pt 1): 287–291. England. doi:10.1111/j.1469-7793.1999.287ad.x.
Ostrowski, K., Rohde, T., Zacho, M., Asp, S., and Pedersen, B.K. 1998b. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol 508 ( Pt 3)(Pt 3): 949–953. England. doi:10.1111/j.1469-7793.1998.949bp.x.
Parsons, J.P. 2010. Current concepts in the diagnosis and management of exercise-induced bronchospasm. doi:10.3810/psm.2010.12.1824.
Parsons, J.P., Craig, T.J., Stoloff, S.W., Hayden, M. lou, Ostrom, N.K., Eid, N.S., and Colice, G.L. 2011. Impact of exercise-related respiratory symptoms in adults with asthma: Exercise-Induced Bronchospasm Landmark National Survey. Allergy Asthma Proc 32(6): 431–437. doi:10.2500/aap.2011.32.3501.
Pedersen, B.K., Ostrowski, K., Rohde, T., and Bruunsgaard, H. 1998. The cytokine response to strenuous exercise. Can J Physiol Pharmacol 76(5): 505–511. Canadian Science Publishing. doi:10.1139/y98-055.
Pedersen, B.K., Steensberg, A., Fischer, C., Keller, C., Keller, P., Plomgaard, P., Febbraio, M., and Saltin, B. 2003. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil 24(2–3): 113–9. doi:10.1023/a:1026070911202.
Pennell, L.M., Galligan, C.L., and Fish, E.N. 2012. Sex affects immunity. J Autoimmun 38(2–3): J282–J291. Elsevier BV. doi:10.1016/j.jaut.2011.11.013.
Peters, H.P.F., Ph, D., Bos, M., Sc, M., Seebregts, L., and Sc, M. 1999. <Gi sysmptoms in long distance runners.pdf>. 94(6).
Pfeiffer, B., Stellingwerff, T., Hodgson, A.B., Randell, R., Pöttgen, K., Res, P., and Jeukendrup, A.E. 2012. Nutritional intake and gastrointestinal problems during competitive endurance events. Med Sci Sports Exerc 44(2): 344–351. doi:10.1249/MSS.0b013e31822dc809.
Rehrer, N.J., Brouns, F., Beckers, E.J., Frey, W.O., Villiger, B., Riddoch, C.J., Menheere, P.P.C.A., and Saris, W.H.M. 1992. Physiological changes and gastro-intestinal symptoms as a result of ultra-endurance running. Eur J Appl Physiol Occup Physiol 64(1): 1–8. Springer Science and Business Media LLC. doi:10.1007/bf00376431.
Riddoch, C., and Trinick, T. 1988. Gastrointestinal disturbances in marathon runners. Br J Sports Med 22(2): 71–4. doi:10.1136/bjsm.22.2.71.
Rogers, C.J., Colbert, L.H., Greiner, J.W., Perkins, S.N., and Hursting, S.D. 2008. Physical Activity and Cancer Prevention. Sports Medicine 38(4): 271–296. Springer Science and Business Media LLC. doi:10.2165/00007256-200838040-00002.
Sarma, J., Laan, C.A., Alam, S., Jha, A., Fox, K.A.A., and Dransfield, I. 2002. Increased Platelet Binding to Circulating Monocytes in Acute Coronary Syndromes. Circulation 105(18): 2166–2171. Ovid Technologies (Wolters Kluwer Health). doi:10.1161/01.cir.0000015700.27754.6f.
Shi, F.-D., Ljunggren, H.-G., la Cava, A., and van Kaer, L. 2011. Organ-specific features of natural killer cells. Nat Rev Immunol 11(10): 658–671. England. doi:10.1038/nri3065.
Smith, L.L. 1991. Acute inflammation. Med Sci Sports Exerc 23(5): 542???551. Ovid Technologies (Wolters Kluwer Health). doi:10.1249/00005768-199105000-00006.
Sossdorf, M., Otto, G.P., Claus, R.A., Gabriel, H.H.W., and Lösche, W. 2011. Cell-derived microparticles promote coagulation after moderate exercise. Med Sci Sports Exerc 43(7): 1169–1176. doi:10.1249/MSS.0b013e3182068645.
Sprang, S.R., and Fernando Bazan, J. 1993. Cytokine structural taxonomy and mechanisms of receptor engagement. Curr Opin Struct Biol 3(6): 815–827. Elsevier BV. doi:10.1016/0959-440x(93)90144-a.
Steensberg, A., Fischer, C.P., Keller, C., Møller, K., and Pedersen, B.K. 2003. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. American Journal of Physiology-Endocrinology and Metabolism 285(2): E433–E437. American Physiological Society. doi:10.1152/ajpendo.00074.2003.
Steensberg, A., Keller, C., Starkie, R.L., Osada, T., Febbraio, M.A., and Pedersen, B.K. 2002. IL-6 and TNF-α expression in, and release from, contracting human skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism 283(6): E1272–E1278. American Physiological Society. doi:10.1152/ajpendo.00255.2002.
Takeda, K., Kaisho, T., and Akira, S. 2003. Toll-Like Receptors. Annu Rev Immunol 21(1): 335–376. Annual Reviews. doi:10.1146/annurev.immunol.21.120601.141126.
Timmons, B.W., Tarnopolsky, M.A., Snider, D.P., and Bar-Or, O. 2006. Immunological changes in response to exercise: influence of age, puberty, and gender. Med Sci Sports Exerc 38(2): 293–304. doi:10.1249/01.mss.0000183479.90501.a0.
Toft, A.D., Jensen, L.B., Bruunsgaard, H., Ibfelt, T., Halkjær-Kristensen, J., Febbraio, M., and Pedersen, B.K. 2002. Cytokine response to eccentric exercise in young and elderly humans. American Journal of Physiology-Cell Physiology 283(1): C289–C295. American Physiological Society. doi:10.1152/ajpcell.00583.2001.
Tsivitse, S.K., McLoughlin, T.J., Peterson, J.M., Mylona, E., McGregor, S.J., and Pizza, F.X. 2003. Downhill running in rats: influence on neutrophils, macrophages, and MyoD+ cells in skeletal muscle. Eur J Appl Physiol 90(5–6): 633–638. Springer Science and Business Media LLC. doi:10.1007/s00421-003-0909-0.
Wang, J.-S., Li, Y.-S., Chen, J.-C., and Chen, Y.-W. 2005. Effects of Exercise Training and Deconditioning on Platelet Aggregation Induced by Alternating Shear Stress in Men. Arterioscler Thromb Vasc Biol 25(2): 454–460. Ovid Technologies (Wolters Kluwer Health). doi:10.1161/01.atv.0000151987.04607.24.
Weiss, C., Seitel, G., and Bartsch, P. 1998. Coagulation and fibrinolysis after moderate and very heavy exercise in healthy male subjects. Medicine &amp Science in Sports &amp Exercise 30(2): 246–251. Ovid Technologies (Wolters Kluwer Health). doi:10.1097/00005768-199802000-00012.
Wright, H., Collins, M., Villiers, R. de, and Schwellnus, M.P. 2011. Are splanchnic hemodynamics related to the development of gastrointestinal symptoms in Ironman triathletes? A prospective cohort study. Clin J Sport Med 21(4): 337–43. doi:10.1097/JSM.0b013e31822148b8.
Wright, H.L., Moots, R.J., Bucknall, R.C., and Edwards, S.W. 2010. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 49(9): 1618–1631. Oxford University Press (OUP). doi:10.1093/rheumatology/keq045.
Ziegler, T.R. 1992. Clinical and Metabolic Efficacy of Glutamine-supplemented Parenteral Nutrition after Bone Marrow Transplantation. Ann Intern Med 116(10): 821. American College of Physicians. doi:10.7326/0003-4819-116-10-821.
Meet the Author
Kevin J. Milne, Department of Kinesiology, University of Windsor

Dr. Kevin Milne is an Associate Professor in the Department of Kinesiology in the Faculty of Human Kinetics at the University of Windsor where he teaches courses in exercise physiology physiology, endocrinology and environmental physiology. He has published on the stress response to exercise, exercise preconditioning, and hormonal influences on human health and performance. He is an active member of the Centre for Human Performance and Health at Windsor and he regularly supervises graduate students in the general areas of exercise physiology physiology and applied human performance. Dr. Milne lives in Windsor, Ontario, he is the father of four daughters, and he maintains an active lifestyle of basketball, soccer, and road running.

