1.2 What Is Reprocessing?
Healthcare providers require instruments and medical devices to perform most procedures. Although some devices are single-use and are discarded after use, it is often more cost-effective for a healthcare facility to purchase reusable medical devices. Medical devices have become increasingly more complex and therefore require more specialized cleaning, disinfection, and sterilization processes.
Reprocessing involves many steps and begins at the point of use—in the procedure room, operating theatre, or at the patient’s bedside. At point of use, gross soil is removed, and devices are packaged for transport. Devices are then transported to the Medical Device Reprocessing Area (MDRA), where medical device reprocessing technicians (MDRT) further clean the devices using a variety of methods. Devices are disinfected to a level that allows personnel to handle them without risking infection. MDRTs then inspect and package the devices before they are sent for sterilization. Finally, the sterile devices are stored until they are needed again.
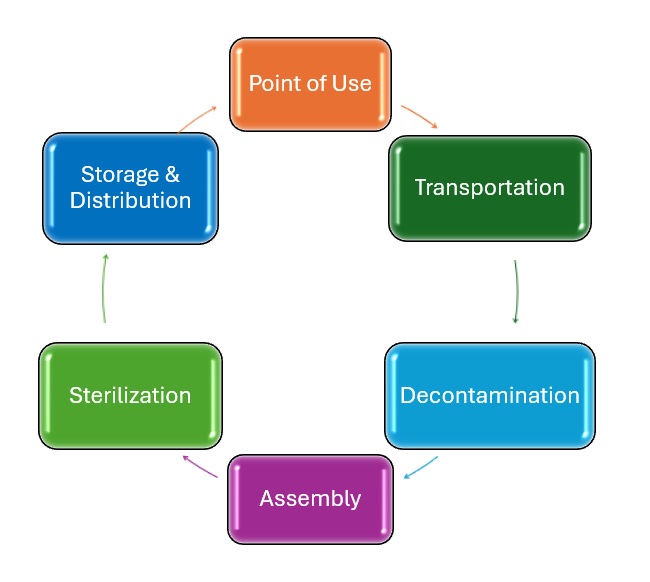
The video below was created by Alberta Health Services (2014) and discusses not just the MDRT profession, but also many key aspects of working in a centralized medical device reprocessing area.
(Alberta Health Services, 2014)
To further your understanding of working in an MDRA, watch the video below as well. It was also created by Alberta Health Services, but the focus is on decentralized reprocessing areas that are located outside the centralized reprocessing department. The reprocessing of medical devices in decentralized locations includes equipment such as surgical or dental instruments, endoscopes, and diagnostic probes.
(Alberta Health Services, n.d.)
What is a medical device?
There are many types of medical devices, and they have been defined as “any instrument or component used to treat, diagnose, or prevent a disease or abnormal physical condition” (Government of Canada, 2015). In Canada, medical devices are classified based on their risk to the health and safety of the patient. There are four classifications: Class I, II, III, and IV. Class I medical devices pose the least risk to the patient and include items such as thermometers and blood pressure cuffs. Class IV medical devices pose the highest risk to patients and include orthopedic implants and pacemakers. The MDRA in a large facility may reprocess devices from all four classifications, but the MDRA most commonly reprocesses items from Classes II, III, and IV.
Spaulding Classification System
In 1957, Dr. E. H. Spaulding introduced a classification system for medical devices based on the infection risk posed by the use of the device. Although the classification criteria have been modified over the years, this system is still in use today to determine the level of reprocessing required for medical devices. Dr. Spaulding proposed three different classifications for medical devices: non-critical, semi-critical, and critical.
Key Concept
There are four “levels” of reprocessing:
- Low-level disinfection
- Intermediate-level disinfection
- High-level disinfection
- Sterilization
These four levels of reprocessing are increasingly more lethal to microorganisms, with sterilization being the complete inactivation of all the microorganisms on a device. All medical devices, as per the Spaulding Classification System, will fit within one of these four levels of reprocessing based on their infection risk and equipment use or characteristics.
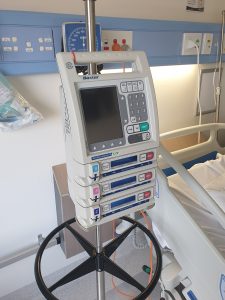
Non-critical medical devices are devices such as IV pumps (Figure 1.2), blood pressure cuffs (Figure 1.3), and stethoscopes. These devices are used only on intact skin and pose the lowest risk of infection to the patient. Devices in this classification require low-level or intermediate-level disinfection.

Semi-critical devices come into contact with non-intact skin or mucous membranes. Flexible colonoscopes (Figure 1.4) and respiratory devices fall into this category. Semi-critical devices require a minimum of high-level disinfection.

Important Takeaway
Although semi-critical devices only require high-level disinfection, sterilization is required if the manufacturer provides sterilization instructions.
Critical medical devices are those that enter the bloodstream or are used in other sterile body cavities such as the lungs or bladder. Devices in this classification include surgical instruments and bronchoscopes (Figure 1.5). Dental and foot care instruments also fall into this category. Critical medical devices must be sterilized.

References
Alberta Health Services. (n.d.). Introduction to decentralized medical device reprocessing [Video]. https://publicshare.albertahealthservices.ca/teams/CDN/AHS_Website/Information_For/if-hp-ipc-ahs-decentralized-1.mp4
Alberta Health Services. (2014, August 13). AHS careers – Medical device reprocessor [Video]. YouTube. https://www.youtube.com/watch?v=Y5SG4RLMzvI&t=3s
Government of Canada. (2015). Guidance document – Guidance on the risk-based classification system for non-in vitro diagnostic Devices (non-IVDDs). https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/application-information/guidance-documents/guidance-document-guidance-risk-based-classification-system-non-vitro-diagnostic.html#a22
Image Credits
(Images are listed in order of appearance)
Erickson, S. (2025). Reprocessing cycle [Diagram]. NorQuest College.
Baxter Colleague 3 CXE 1 at Joondalup 2020 by Orderinchaos, CC BY-SA 4.0
For measuring blood pressure by Paolocmartin, CC BY-SA 4.0
Colonoscope by Gilo1969, CC BY-SA 3.0
Bronkoskop by Håkon Olav Leira, CC BY-SA 3.0

