6.2 What is a Quality Management System?
Quality in healthcare is the degree to which a healthcare setting is able to meet the needs of clients and provide the desired health outcomes for patients (World Health Organization [WHO], 2020). In the Medical Device Reprocessing Area (MDRA), it is essential to provide a quality product in order to prevent infection and make sure that the correct devices are available and ready when needed. A quality management system (QMS) includes tracking, testing, policies, and procedures that are put into place to ensure all products meet the requirements of the healthcare organization as well as the needs of the clients.
The main goal of the QMS is to ensure that all processes are performed the same way every time, providing consistent results. The control of processes guarantees a quality product that meets the needs and expectations of the users and meets the requirements of governing standards, guidelines, and laws.
Key Concept
Any healthcare facility in Canada that reprocess medical devices uses standards and guidelines from the following organizations:
- Canadian Standards Association (CSA) (2023) publishes the standard CSA Z314:23 – Canadian medical device reprocessing in all healthcare settings. This standard is the source for most procedures in the MDRA and must be followed in any facility performing invasive procedures (surgeries).
- Accreditation Canada (AC) (2025) standards were developed based on standards from the International Standards Organization (ISO). AC conducts regular audits of healthcare facilities in Canada and provides accreditation to facilities based on audit results.
- Provincial and federal infection prevention guidelines provide facilities with guidance on preventing infection transmission. Recalls and product alerts are issued by these organizations. Federal guidelines are issued by the Public Health Agency of Canada (PHAC).
- User groups such as the Operating Room Nurses Association of Canada (ORNAC), the Association for the Advancement of Medical Instrumentation (AAMI), and the Canadian Association of Gastroenterology provide recommendations for medical device reprocessing.
QMS Framework
All healthcare settings in Canada that reprocess medical devices are required to create and maintain a quality management system. The required framework for the QMS is outlined in the CSA Z314-23 standard and is based on international standards (Canadian Standards Association, 2023). The QMS will address planning, supports, orientation, operation, leadership, evaluation of process performance, and improvement. Elements of the QMS framework include policies, standard operating procedures (SOPs), personnel, leadership/management, and process documentation.
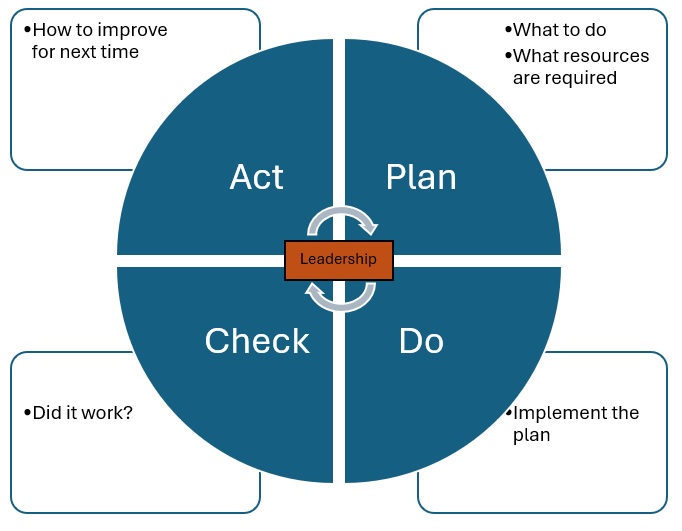
Plan-Do-Check-Act
The framework for the MDRA QMS uses the Plan-Do-Check-Act (PDCA) model (Figure 6.1), which is used widely across the world. This model ensures that all areas of the MDRA are captured in the QMS. Leadership is responsible for utilizing the model.
- Plan: Planning involves deciding what activities need to be done or improved upon, then deciding what to do and what resources are required to move forward with the plan.
- Do: This is where the plan created in the first step is implemented, including how the plan will be carried out, who will be responsible for the tasks in the plan, and where and when the plan will be implemented.
- Check: This is stage at which leadership will evaluate the activities and decide whether the plan has achieved the intended results.
- Act: In this phase of the model, leadership will evaluate how the process can be improved upon for the future. This phase leads back into the Plan phase of the model.
(Canadian Standards Association, 2023)
References
Accreditation Canada. (2025). People powered health. https://accreditation.ca/
Canadian Standards Association (CSA). (2023). CSA Z314:23 – Canadian medical device reprocessing in all health care settings. CSA Group. https://www.csagroup.org/store/product/2430344/
World Health Organization (WHO). (2020, July 20). Quality health services. https://www.who.int/news-room/fact-sheets/detail/quality-health-services
Image Credit
Erickson, S. (2025). Plan-Do-Check-Act [Diagram]. NorQuest College.

