1.4 One-Way Workflow
The direction of the workflow in the MDRA is very important in preventing the contamination of sterile or disinfected devices. Devices must travel through the MDRA from the dirtiest area to the cleanest. This is called a one-way workflow.
Key Concept
A one-way workflow is much like a one-way street. If a car drives the wrong way down a one-way street, an accident could occur. Similarly, if a soiled device enters the sterile storage area of the MDRA, contamination of a sterile device could occur.
The design and layout of the MDRA is critical in maintaining the one-way workflow and is shown in Figure 1.21. Soiled devices are received in the Decontamination area, where they are cleaned and disinfected. Devices are then brought to the Assembly area, where they are inspected for functionality and cleanliness, packaged, and labelled. Next is Sterilization, where the packaged devices are sterilized and cooled. Finally, the devices are stored in the Storage & Distribution area until they are needed.
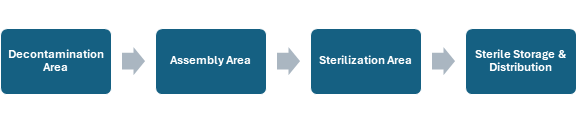
The level of disinfection or sterility of a device increases as it moves through the different areas of the MDRA. Any breaks in the one-way workflow risks cross-contamination.
The One-Way Workflow Process
Decontamination Area
The decontamination area is at the beginning of the MDRA workflow. Its main purpose is to clean and disinfect devices to reduce the microorganisms on a device and allow personnel on the “clean” side to handle the devices without gloves.
The Decontamination area must be physically separated from the rest of the MDRA to prevent the contamination of clean devices and is shown in Fig. 1.22.. The areas are kept separate using walls, pass-through windows, and pass-through washer-disinfectors.
After a procedure, the reusable medical devices are transported to the Decontamination area of the MDRA. The contaminated medical devices are sorted, disassembled as needed, then cleaned (Fig. 1.23). Gross soil is removed from the device using manual friction or mechanical cleaning as recommended by the device manufacturer.


To work in this area, MDRTs must have thorough knowledge of how to disassemble, clean, and disinfect many different types of devices. Cleaning and disinfection requires knowledge of the use of many different chemicals, detergents, and equipment, as well as different cleaning methods. Knowledge of infection prevention principles, sharps safety, blood and body fluid exposure prevention, WHMIS, and proper body mechanics are essential for an MDRT to keep themselves and others safe while working in the Decontamination area.
Assembly Area
The second area in the MDRA one-way workflow is assembly, also known as “prep and pack.” It is where devices that have undergone cleaning and disinfection are inspected for cleanliness and function, then packaged and labelled for sterilization. The Assembly area, as shown in Figures 1.24 and 1.25, receives clean devices from the Decontamination area. The devices are then sorted and brought to workstations to be prepared for sterilization.
MDRTs working in the Assembly area must have knowledge of multiple different types of surgical instruments and other medical devices and how to prepare them for sterilization. Medical devices must be carefully inspected for any remaining soil or damage and tested to ensure proper function. MDRTs are trained to use different tools to inspect and test devices, including magnifiers and borescopes.


Following inspection and testing, devices must be packaged for sterilization. Packaging systems include reusable rigid sterilization containers, peel packs, and reusable or disposable sterilization wrap. An MDRT must know how to properly inspect and assemble rigid sterilization containers and be familiar with wrapping methods.
Devices are then labelled to indicate what is inside each package. The label will identify the package contents and the identity of the person who assembled the set. Some labels will also include the storage location and sterilization method.
Sterilization Area
Once devices are prepared, they are brought to the sterilization area, where devices are exposed to steam or chemical sterilants that deactivate or kill all the microorganisms on the devices.
Steam sterilization is the most commonly used sterilization method and is performed using steam sterilizers that can withstand the high pressure created during a steam sterilization cycle. MDRTs must understand how steam sterilizers operate and how to interpret cycle parameter readings.
Low-temperature sterilization methods use chemicals to kill microorganisms. These sterilization methods were developed to sterilize medical devices that would be damaged by steam. It is essential that an MDRT know how to operate low-temperature sterilizers, interpret cycle parameters, and be familiar with the safety requirements for chemical sterilants.
MDRTs have a legal responsibility to document all sterilization cycles. The documentation allows the healthcare facility to demonstrate that the criteria for sterilization has been met. All devices that are sterilized are recorded and traceable in the event that a sterilization failure is identified. Sterility assurance tests are performed and documented to provide an extra measure of quality assurance.
Sterile Storage & Distribution Area
The sterile storage & distribution area is where sterile, packaged devices are stored until they are needed for a procedure or surgery. This area requires specific storage conditions to ensure that devices remain sterile until they are used.
MDRTs working in this area are required to handle packaged devices in a manner that prevents contamination of the contents. Knowledge of sterility maintenance concepts is essential. MDRTs must know how to identify devices whose sterility has been compromised.
The Sterile Storage area has restricted access to prevent the introduction of outside contaminants. High amounts of traffic increase the amount of dust and air movement in the area. The temperature and humidity is monitored and controlled. Personnel entering the area must perform hand hygiene and don proper attire such as gowns to cover clothing and caps to confine hair. Outside clothing or footwear is prohibited.
When the operating room (OR) books a procedure, the MDRA is provided with a list of devices needed for the procedure. An MDRT will then pull the devices from the storage shelves and put them into a case cart to transport to the OR. MDRTs will also prepare and distribute sterile supplies throughout the facility.
Potential Breaches
Some examples of how breaches to one-way workflow procedures can occur in different areas of the MDRA:
- If a sterile instrument set is brought into Decontamination, it must be fully reprocessed.
- If a dirty device is found in the Assembly area, the device must be returned to the Decontamination area for cleaning and the workstation cleaned and disinfected.
- If a soiled instrument set is mistakenly put away in Sterile Storage, the soiled set and neighbouring sets are returned to decontamination for reprocessing and the storage shelving cleaned and disinfected.
The MDRA is an essential part of any healthcare environment where invasive procedures are performed. The layout and design of the MDRA is critical to preventing the cross-contamination of devices during reprocessing. MDRTs must possess significant amounts of knowledge to perform all the required duties.
Image Credits
(Images are listed in order of appearance)
Erickson, S. (2025). One-way workflow [Diagram]. NorQuest College.
Willekes, C. (2025). MDRA decontamination area, University of Alberta Hospital [Photo]. NorQuest College.
Willekes, C. (2025). MDRA decontamination area, University of Alberta Hospital [Photo]. NorQuest College.
Willekes, C. (2025). MDRA assembly area, University of Alberta Hospital [Photo]. NorQuest College.
Willekes, C. (2025). MDRA assembly area, University of Alberta Hospital [Photo]. NorQuest College.

