Henderson-Hasselbalch equation
An equation that allows you to calculate the proportions of a drug (either a weak acid or a weak base) that exists in charged and uncharged forms. It is necessary to know whether the drug is a weak acid (becomes more protonated and more uncharged as pH drops) or a weak base (becomes more protonated and more charged as pH drops), as well as the pKa for the compound (the pH at which 50% of the drug is ionised), in order that the correct form of the equation is used.
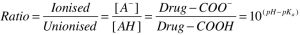
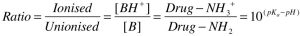
This is important because only uncharged drug can cross cell membranes; in the clinic, altering urinary pH is a strategy to increase renal clearance of a drug (through reducing reabsorption, by increasing the proportion of charged drug present in the urine) following an overdose. A weak acid or base causing serious CNS side effects can also be “pulled out” of the brain by changing the plasma pH such that the drug is more ionised in the plasma. This would also have the effect of reducing the apparent volume of distribution for the drug.

