Dissociation
Dissociation refers to the departure of a ligand from its binding site on a protein. The rate of dissociation is governed by a dissociation rate constant. The more tightly a ligand binds to its binding site, the longer the ligand remains bound after it associates with the receptor before it dissociates from the receptor.
Dissociation refers specifically to the breaking-apart of a complex LP to yield a free ligand (L) and a free receptor or other target protein (P):
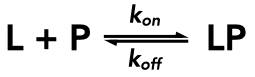
The rate of dissociation is governed by a dissociation rate constant, referred to here as koff, but often also as k-1. The units of koff, a first-order rate constant, for the dissociation event are s-1. The value of koff indicates the proportion of the LP complexes that would dissociate to yield L and P in unit time if the rate of dissociation at the time of interest was maintained for the entire time period in question. That being said, the dissociation rate becomes slower as [LP] falls, and a plot of [LP] versus time would be exponential.
The rate of the reverse reaction (yielding L and P) at any point in time is found by multiplying the concentration of the complex, [LP], remaining at that time by the dissociation rate constant. Thus, rate = koff[LP]. A half-life for dissociation of the complex can be found from ln 2/koff, while 1/koff yields a value for the mean target residence time – the average time for which a ligand molecule remains bound to the target before dissociating.
Further details and examples may be found under the entry for affinity.

