Area under the curve
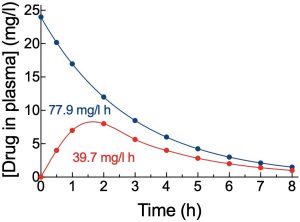
This usually refers to the area under the curve (AUC) for a graph of plasma concentration versus time. The units of AUC are the Y-axis units (units of concentration) multiplied by the X-axis units (units of time); for example, (mg/l)h or (µg/ml)min. There are several ways to calculate AUC, including through use of software, or manually by applying the trapezoidal rule or by a cut-and-weigh technique.
It may be necessary to determine a drug’s AUC in order to calculate oral bioavailability (discussed below), or to determine the non-compartmental apparent volume of distribution (AVD area).
In a single individual, the AUC is proportional to the proportion of the dose of drug that is absorbed into the systemic circulation, and the actual value is impacted by the amount of the drug that is absorbed, and by how rapidly it is then removed. As such, a comparison of an IV dose with a PO dose of the same drug in the same individual on two different occasions will provide two AUC values that allow determination of oral bioavailability (from AUCPO/AUCIV). This is because the drug is removed with similar clearance values regardless of the route by which it is administered and the only significant difference between areas under the two curves would be caused by differences in the amount of drug absorbed into the circulation. In the same way, AUC for the same dose of the same drug by the same route in an individual may change if ADME parameters change for the drug in that individual – e.g. altered bioavailability due to a change in Clint for a high extraction ratio drug, altered clearance due to a change in Clint for a low extraction ratio drug, etc. In the latter example of altered clearance, note that AUC values for PO and IV administration routes in that individual would both change to a similar degree and so bioavailability would not be substantially affected (see example calculation box).
Example calculation
Drug X is cleared entirely by hepatic metabolism and is a low extraction ratio drug. ClT for drug X is 5 l/h. After an IV dose of 500 mg, the AUC is 100 (mg/l)h (from Cl=Dose/AUC). A 500 mg PO dose gives an AUC of 50 (mg/l)h. Oral bioavailability (F) is therefore 50/100 = 50%. If the patient now drinks grapefruit juice and Clint is reduced by 80% then clearance is reduced to 1 l/h. As a result, the AUC following an IV dose of 500 mg increases to 500 (mg/l)h and the AUC following a PO dose increases to 250 (mg/l)h. But bioavailability would not have changed as it would now equal 250/500 = 50%.
In an intravenous repeated dosing regimen that has reached a steady state concentration, the average concentration in the plasma can be calculated from the AUC between two consecutive doses, divided by the time interval between doses. This average concentration typically lies slightly closer to the trough concentration than to the peak concentration, with this tendency becoming more marked as the time interval between doses becomes larger.
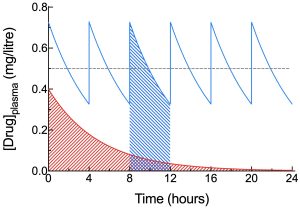
At steady state, it stands to reason that the amount of drug lost from the body between consecutive doses is identical to the amount of drug administered in each dose. As such, the AUC between two consecutive doses at steady state should be similar to the AUC from t=0 to t=∞ (AUC0-∞) after a single IV dose of the drug (because one full dose of the drug is also lost from the body in such a scenario), assuming the size of the dose is the same in both cases, and assuming there has been no change in drug clearance (as a result of a change in intrinsic clearance, for example) during chronic drug dosing required to reach steady state. This is illustrated in the figure on the right, where the areas highlighted in red and blue hatching are identical in size. Under the entry for clearance, it is shown that total body clearance, ClT, may be calculated from ClT = Dose/AUC0-∞. Based on the discussion above, it should be apparent that it is also possible to calculate total body clearance if the AUC between two consecutive doses of drug is known, since this value should be similar to AUC0-∞. The equation then becomes ClT = Dose/AUC0-τ, where t=0 represents the time at which the first of two consecutive doses is given, and t=τ represents a later time at which the subsequent dose is administered, with the dosing interval being equal to τ.

